Structural and physico-chemical properties of selected glasses in the (Ca1-xMgx)O-Al2O3 system
Abstract
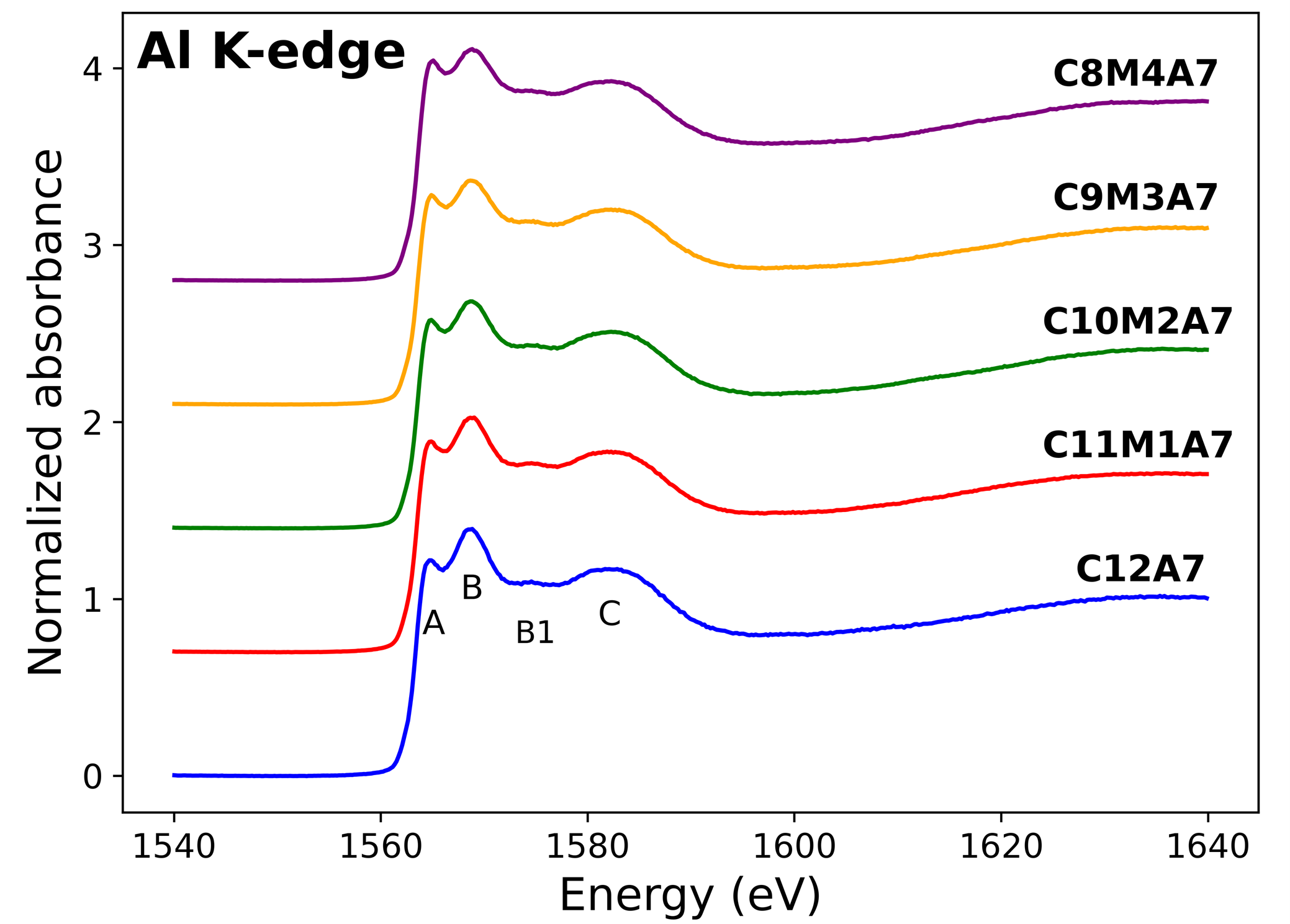
Oxide glasses and melts are of interest in many fields of science and technology. The structural and physico-chemical properties of C12A7, C11M1A7, C10M2A7, C9M3A7, and C8M4A7 glasses (C = CaO, M = MgO and A = Al2O3) were studied for the first time. It was shown by Al K-edge XANES spectroscopy that Al is in fourfold coordination in all studied glass samples with increasing Q4/Q2 ratio in the C12A7-C8M4A7 composition range. The coordination number of Ca was found to be between 6 and 7 in all studied glasses by Ca K-edge XANES spectroscopy. From the thermodynamic point of view there is no composition dependence, within experimental uncertainty, of the heat capacity in the C12A7-C8M4A7 composition range. In contrast, the enthalpy of formation from the constituent solid oxides at 298.15 K was found to be greater for C8M4A7 than for C12A7 glasses. A decrease of density, molar volume and glass transition temperature was observed for increasing MgO content. In addition to the experimental investigation, classical molecular dynamics simulations were conducted using a recently optimized Born-Mayer-Huggins potential. It was found that the simulations present a good estimation of the structural properties (local surroundings of Ca and Mg) and some of the thermodynamic properties (heat capacity of glasses and liquids), but overestimate the values of the coordination number of Al and the other thermodynamic properties (density and enthalpy of formation from oxides at 298.15 K).
Keywords
Full Text:
PDFReferences
Pashtoon MI. Miakhil S, Behsoodi MM. Waste Glass Powder “An Alternative of Cement in Concrete”: A Review. Int J Curr Res Rev. 2022;05:2541-9. doi:10.47191/ijcsrr/V5-i7-39
El-Sayed Seleman MM, El-kheshen AA, Kharbish S, Ebrahim WR. Utilization of Cement Kiln Dust for the Preparation of Borosilicate Glass. Interceram Int Ceram Rev. 2020;69:26–33. doi:10.1007/s42411-020-0426-8
Shearer A, Hauke B, Montazerian M, Mauro JC. A critical review of infrared transparent oxide glasses. Opt Mater X. 2023;20:100258. doi:10.1016/j.omx.2023.100258
de Araújo CB, Kassab LRP, da Silva DM. Optical properties of glasses and glass-ceramics for optical amplifiers, photovoltaic devices, color displays, optical limiters, and Random Lasers. Opt Mater. 2022;131:112648. doi:10.1016/j.optmat.2022.112648
Alzahrani AS. A Review of Glass and Crystallizations of Glass-Ceramics. Adv Mater Phys Chem. 2022;12:261–88. doi:10.4236/ampc.2022.1211018
Almendro-Candel MB, Jordán Vidal MM. Glasses, Frits and Glass-Ceramics: Processes and Uses in the Context of Circular Economy and Waste Vitrification. Coatings. 2024;14:346. doi:10.3390/coatings14030346
Du P, Yuan P, Liu J, Ye B. Clay minerals on Mars: An up-to-date review with future perspectives. Earth Sci Rev. 2023;243:104491. doi:10.1016/j.earscirev.2023.104491
Musgraves JD, Hu J, Calvez L, eds. Springer Handbook of Glass. Springer Nature; 2019. 1841p.
Neuville DR, Henderson GS, Dingwell DB, eds. Geological melts. Vol. 87. Walter de Gruyter GmbH & Co KG; 2022. 1088p.
McMillan P, Piriou B. Raman spectroscopy of calcium aluminate glasses and crystals. J Non Cryst Solids. 1983;55:221–42. doi:10.1016/0022-3093(83)90672-5
Neuville DR, Henderson GS, Cormier L, Massiot D. The structure of crystals, glasses, and melts along the CaO-Al2O3 join: Results from Raman, Al L- and K-edge X-ray absorption, and 27Al NMR spectroscopy. Am Mineral. 2010;95:1580–9. doi:10.2138/am.2010.3465
Neuville DR, Cormier L, de Ligny D, Roux J, Flank A-M, Lagarde P. Environments around Al, Si, and Ca in aluminate and aluminosilicate melts by X-ray absorption spectroscopy at high temperature. Am Mineral. 2008;93:228–34. doi:10.2138/am.2008.2646
Neuville DR, Cormier L, Flank A-M, Briois V, Massiot D. Al speciation and Ca environment in calcium aluminosilicate glasses and crystals by Al and Ca K-edge X-ray absorption spectroscopy. Chem Geol. 2004;213:153–63. doi:10.1016/j.chemgeo.2004.08.039
Hallstedl B. Assessment of the CaO-Al2O3 System. J Am Ceram Soc. 1990;73:15–23. doi:10.1111/j.1151-2916.1990.tb05083.x
Richet P, Nidaira A, Neuville DR, Atake T. Aluminum speciation, vibrational entropy and short-range order in calcium aluminosilicate glasses. Geochim Cosmochim Acta. 2009;73:3894–904. doi:10.1016/j.gca.2009.03.041
Arkhipin AS, Pisch A, Zhomin GM, Kuzovchikov S V, Khvan A V., Smirnova NN, Markin AV, Kovalenko NA, Uspenskaya IA. Thermodynamic properties of selected glasses in the CaO–Al2O3–TiO2 system. J Non Cryst Solids. 2023;603:122098. doi:10.1016/j.jnoncrysol.2022.122098
Navrotsky A, Peraudeau G, McMillan P, Coutures J-P. A thermochemical study of glasses and crystals along the joins silica-calcium aluminate and silica-sodium aluminate. Geochim Cosmochim Acta. 1982;46:2039–47. doi:10.1016/0016-7037(82)90183-1
Abel BM, Mauro JC, Smedskjaer MM, Morgan JM, Lapierre CL, Swan GR, Mack ME, Ellison AJ. Liquidus surface of MgO-CaO-Al2O3-SiO2 glass-forming systems. J Non Cryst Solids. 2013;363:39–45. doi:10.1016/j.jnoncrysol.2012.12.020
Hamdan A, Hajimohammadi A, Rawal A, Kim T. The intrinsic role of network modifiers (Ca versus Mg) in the reaction kinetics and microstructure of sodium silicate-activated CaO-MgO-Al2O3-SiO2 glasses. Cem Concr Res. 2023;164:1–16. doi:10.1016/j.cemconres.2022.107058
Veit U, Rüssel C. Density of quaternary glasses in the MgO-CaO-Al2O3-SiO2-system—modeling vs measurement. Int J Appl Glass Sci. 2017;8:301–12. doi:10.1111/ijag.12263
Oprea C, Togan D, Popescu C. Structure and properties of glasses with a low amount of SiO2 in a quaternary system of Al2O3-SiO2-CaO-MgO. Thermochim Acta. 1992;194:165–73. doi:10.1016/0040-6031(92)80015-O
Mongalo L, Lopis AS, Venter GA. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO–MgO–Al2O3–SiO2 melts. J Non Cryst Solids. 2016;452:194–202. doi:10.1016/j.jnoncrysol.2016.08.042
Nie S, Thomsen RM, Skibsted J. Impact of Mg substitution on the structure and pozzolanic reactivity of calcium aluminosilicate (CaO-Al2O3-SiO2) glasses. Cem Concr Res. 2020;138:1–16. doi:10.1016/j.cemconres.2020.106231
Veit U, Rüssel C. Viscosity and liquidus temperature of quaternary glasses close to an eutectic composition in the CaO–MgO–Al2O3–SiO2 system. J Mater Sci. 2017;52:8280–92. doi:10.1007/s10853-017-1044-3
Neuville DR, Cormier L, Montouillout V, Florian P, Millot F, Rifflet J-C, Massiot D. Structure of Mg- and Mg/Ca aluminosilicate glasses: 27Al NMR and Raman spectroscopy investigations. Am Mineral. 2008;93:1721–31. doi:10.2138/am.2008.2867
Sugimura T, Deura T, Sakamoto K, Sukenaga S, Saito N, Nakashima K. Effect of Li2O Addition on Crystallization Behavior of CaO–Al2O3–MgO Based Inclusions. ISIJ International. 2011;51:1982–6. doi:10.2355/isijinternational.51.1982
Kucharczyk S, Zajac M, Stabler C, Thomsen RM, Ben Haha M, Skibsted J, Deja J. Structure and reactivity of synthetic CaO-Al2O3-SiO2 glasses. Cem Concr Res. 2019;120:77–91. doi:10.1016/j.cemconres.2019.03.004
Zajac M, Skocek J, Lothenbach B, Mohsen BH. Late hydration kinetics: Indications from thermodynamic analysis of pore solution data. Cem Concr Res. 2020;129:105975. doi:10.1016/j.cemconres.2020.105975
Farges F, Neuville DR, Brown GE. Structural investigation of platinum solubility in silicate glasses. Am Mineral. 1999;84:1562–8. doi:10.2138/am-1999-1009
Newville M. Larch: An Analysis Package for XAFS and Related Spectroscopies. J Phys Conf Ser. 2013;430:012007. doi:10.1088/1742-6596/430/1/012007
Richet P, Whittington A, Holtz F, Behrens H, Ohlhorst S, Wilke M. Water and the density of silicate glasses. Contrib Mineral Petrol. 2000;138:337–47. doi:10.1007/s004100050567
Standard Material 720, Synthetic Sapphire (α-Al2O3), National Bureau of Standards. 1982.
Jakse N, Alvares CMS, Pisch A. Ab initio based interionic interactions in calcium aluminotitanate oxide melts: structure and diffusion. J Phys Condens Matter. 2021;33:285401. doi:10.1088/1361-648X/abfc0f
Arkhipin AS, Pisch A, Uspenskaya IA, Jakse N. A Molecular Dynamics Simulation Study of Crystalline and Liquid MgO. Ceramics. 2024;7:1187–203. doi:10.3390/ceramics7030078
Gissinger JR, Nikiforov I, Afshar Y, Waters B, Choi M, Karls DS, Stukowski A, Im W, Heinz H, Kohlmeyer A, Tadmor EB. Type Label Framework for Bonded Force Fields in LAMMPS. J Phys Chem B. 2024;128:3282–97. doi:10.1021/acs.jpcb.3c08419
Li D, Bancroft GM, Fleet ME, Feng XH, Pan Y. Al K-edge XANES spectra of aluminosilicate minerals. Am Mineral. 1995;80:432–40. doi:10.2138/am-1995-5-602
Ildefonse P, Cabaret D, Sainctavit P, Calas G, Flank A-M, Lagarde P. Aluminium X-ray absorption Near Edge Structure in model compounds and Earth’s surface minerals. Phys Chem Miner. 1998;25:112–21. doi:10.1007/s002690050093
Cabaret D, Sainctavit P, Ildefonse P, Flank A-M. Full multiple-scattering calculations on silicates and oxides at the Al K edge. J Phys Condens Matter. 1996;8:3691–704. doi:10.1088/0953-8984/8/20/015
Combes JM, Brown Jr GE, Waychunas GA. X-ray absorption study of the local Ca environment in silicate glasses. XAFS VI, Sixth Int’l Conf on X-ray Absorption Fine Structure Edited by SS Hasnain, Ellis Horwood, Chichester, UK. 1991;312–4.
Guignard M, Cormier L. Environments of Mg and Al in MgO–Al2O3–SiO2 glasses: A study coupling neutron and X-ray diffraction and Reverse Monte Carlo modeling. Chem Geol. 2008;256:111–8. doi:10.1016/j.chemgeo.2008.06.008
Shimoda K, Tobu Y, Hatakeyama M, Nemoto T, Saito K. Structural investigation of Mg local environments in silicate glasses by ultra-high field 25Mg 3QMAS NMR spectroscopy. Am Mineral. 2007;92:695–8. doi:10.2138/am.2007.2535
Shimoda K, Tobu Y, Shimoikeda Y, Nemoto T, Saito K. Multiple Ca2+ environments in silicate glasses by high-resolution 43Ca MQMAS NMR technique at high and ultra-high (21.8T) magnetic fields. J Magn Reason. 2007;186:156–9. doi:10.1016/j.jmr.2007.01.019
El Hayek R, Ferey F, Florian P, Pisch A, Neuville DR. Structure and properties of lime alumino-borate glasses. Chem Geol. 2017;461:75–81. doi:10.1016/j.chemgeo.2016.11.025
Alvares CMS, Deffrennes G, Pisch A, Jakse N. Thermodynamics and structural properties of CaO: A molecular dynamics simulation study. J Chem Phys. 2020;152:084503. doi:10.1063/1.5141841
Voronin GF, Kutsenok IB. Universal Method for Approximating the Standard Thermodynamic Functions of Solids. J Chem Eng Data. 2013;58:2083–94. doi:10.1021/je400316m
Glushko VP, Gurvich LV, Bergman GA, Veyts IV, Medvedev VA, Khachkuruzov GA, Yungman VS. Thermodynamic properties of individual substances. Vol. III. Moscow: Nauka; 1981. 471p.
Cheng J, Navrotsky A. Energetics of La1−xAxCrO3−δ perovskites (A=Ca or Sr). J Solid State Chem. 2005;178:234–44. doi:10.1016/j.jssc.2004.11.028
Navrotsky A. Progress and New Directions in Calorimetry: A 2014 Perspective. J Am Ceram Soc. 2014;97:3349–59. doi:10.1111/jace.13278
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, et al. Reprint of: FactSage thermochemical software and databases, 2010–2016. CALPHAD. 2016;55:1–19. doi:10.1016/j.calphad.2016.07.004
DOI: https://doi.org/10.15826/chimtech.2025.12.2.18
Copyright (c) 2025 Anatoly S. Arkhipin, Georgii M. Zhomin, Semen V. Kuzovchikov, Alexandra V. Khvan, Irina A. Uspenskaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







