Copper(II) complexes with fluorinated 5-aryl-2,2’-bipyridine-6(6’)-carboxylic acid tridentate ligands
Abstract
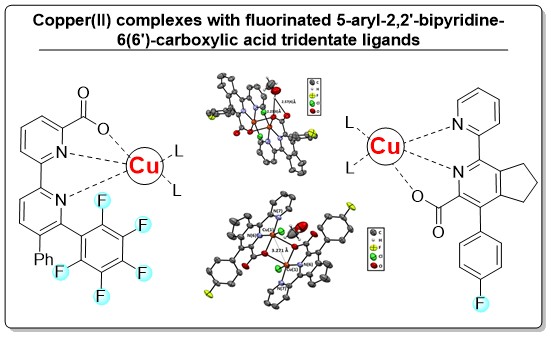
Three Cu(II) complexes containing (poly)fluorine ligands based on 5-aryl-2,2’-bipyridine-6(6’)-carboxylic acids have been synthesized for the first time. These complexes belong to either triclinic (P-1) or orthorhombic (Iba2) crystal systems. The packing of molecules in Cu(II) complexes, confirmed by X-ray diffraction (XRD) structural analysis, seems to be due to the effect of fluorine atoms involved in the formation of hydrogen bonds or intermolecular short contacts of type C–F…π, as well as the influence of the crystallization solvent molecules. The developed coordination compounds could be of interest in the design of pharmacologically active substances, chemosensors, and catalytic systems.
Keywords
Full Text:
PDFReferences
Golubeva YA, Lider EV. Copper(ii) complexes based on 2,2’-bipyridine and 1,10-phenanthroline as potential objects for developing antitumor drugs. J Struct Chem. 2024;65(6):1159–1209. doi:10.1134/s0022476624060088
Dorairaj DP, Kumar P, Rajasekaran H, Bhuvanesh N, Hsu SCN, Karvembu R. Copper(II) complexes containing hydrazone and bipyridine/phenanthroline ligands for anticancer application against breast cancer cells. J Inorg Biochem. 2025;262(112759):112759. doi:10.1016/j.jinorgbio.2024.112759
Shchegolkov EV, Shchur IV, Burgart YV, Slepukhin PA, Evstigneeva NP, Gerasimova NA, Zilberberg NV, Kungurov NV, Saloutin VI, Chupakhin ON. Copper(II) and cobalt(II) complexes based on methyl trifluorosalicylate and bipyridine-type ligands: Synthesis and their antimicrobial activity. Polyhedron. 2021;194(114900):114900. doi:10.1016/j.poly.2020.114900
Korpi H, Sippola V, Filpponen I, Sipilä J, Krause O, Leskelä M, Repo T. Copper-2,2′-bipyridines: Catalytic performance and structures in aqueous alkaline solutions. Appl Catal A Gen. 2006;302(2):250–256. doi:10.1016/j.apcata.2006.01.020
Romo AIB, dos Reis MP, Nascimento OR, Bernhardt PV, Rodríguez-López J, Diógenes ICN. Interplay of electronic and geometric structure on Cu phenanthroline, bipyridine and derivative complexes, synthesis, characterization, and reactivity towards oxygen. Coord Chem Rev. 2023;477(214943):214943. doi:10.1016/j.ccr.2022.214943
Burgart Y, Shchegolkov E, Shchur I, Kopchuk D, Gerasimova N, Borisevich S, Evstigneeva N, Zyryanov G, Savchuk M, Ulitko M, Zilberberg N, Kungurov N, Saloutin V, Charushin V, Chupakhin O. Promising antifungal and antibacterial agents based on 5‐aryl‐2,2′‐bipyridines and their heteroligand salicylate metal complexes: Synthesis, bioevaluation, molecular docking. ChemMedChem. 2022;17(3):e202100577 doi:10.1002/cmdc.202100577
Safin DA, Mitoraj MP, Robeyns K, Filinchuk Y, Vande Velde CML. Luminescent mononuclear mixed ligand complexes of copper(i) with 5-phenyl-2,2′-bipyridine and triphenylphosphine. Dalton Trans. 2015;44(38):16824–16832. doi:10.1039/c5dt02755a
Tong J, Zhao L-R, Zhang J, Wang X-Y, Yu Y-M, Yu S-Y. Heteroleptic copper(i) complexes bearing functionalized 1H-pyrazole-bipyridine ligands: synthesis, photophysical properties, crystal structures, and applications in halogen sensing. New J Chem. 2023;47(9):4374–4385. doi:10.1039/d2nj05408f
Prokhorov AM, Slepukhin PA, Kozhevnikov DN. CuCl2 induced reactions of 6-ethynyl- and 6-cyano-5-aryl-2,2′-bipyridines with various N- and O-nucleophiles in comparison with the reactions of relative 1,2,4-triazines. J Organomet Chem. 2008;693(10):1886–1894. doi:10.1016/j.jorganchem.2008.02.016
Gao D-Z, Sun Y-Q, Liao D-Z, Jiang Z-H, Yan S-P. Synthesis and crystal structures of two metal-nitroxide complexes including a phenanthroline-substituted nitroxide radical. J Coord Chem. 2008;61(15):2413–2421. doi:10.1080/00958970801914082
Maheswari PU, Lappalainen K, Sfregola M, Barends S, Gamez P, Turpeinen U, Mutikainen I, van Wezel GP, Reedijk J. Structure and DNA cleavage properties of two copper(ii) complexes of the pyridine-pyrazole-containing ligands mbpzbpy and Hmpzbpya. Dalton Trans. 2007;33:3676. doi:10.1039/b704390b
Moghimi A, Alizadeh R, Aghabozorg H, Shockravi A, Aragoni MC, Demartin F, Isaia F, Lippolis V, Harrison A, Shokrollahi A, Shamsipur M. Ion pairing, H-bonding, and π–π interactions in copper(II) complex-organo-networks derived from a proton-transfer compound of the 1,10-phenanthroline-2,9-dicarboxylic acid. J Mol Struct. 2005;750(1–3):166–173. doi:10.1016/j.molstruc.2005.04.031
Stipurin S, Strassner T. C^C* platinum(II) complexes with electron-withdrawing groups and beneficial auxiliary ligands: Efficient blue phosphorescent emission. Inorg Chem. 2021;60(15):11200–11205. doi:10.1021/acs.inorgchem.1c01172
Khavasi HR, Rahimi N. Fluorine‐substituted ligands induce structural diversity of coordination compounds. ChemistrySelect. 2017;2(34):11314–11321. doi:10.1002/slct.201702047
Honzíčková I, Vinklárek J, Romão CC, Růžičková Z, Honzíček J. Novel indenyl ligands bearing electron-withdrawing functional groups. New J Chem. 2016;40(1):245–256. doi:10.1039/c5nj02406d
Berger R, Resnati G, Metrangolo P, Weber E, Hulliger J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem Soc Rev. 2011;40(7):3496. doi:10.1039/c0cs00221f
Páez-Franco JC, Zermeño-Ortega MR, de la O-Contreras CM, Canseco-González D, Parra-Unda JR, Avila-Sorrosa A, Enríquez RG, Germán-Acacio JM, Morales-Morales D. Relevance of fluorinated ligands to the design of metallodrugs for their potential use in cancer treatment. Pharmaceutics. 2022;14(2):402. doi:10.3390/pharmaceutics14020402
CrysAlisPro, version 1.171.39.38a, Data Collection, Reduction and Correction Program, Rigaku Oxford Diffraction, 2017.
Sheldrick GM. SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr A Found Adv. 2015;71(1):3–8. doi:10.1107/s2053273314026370
Sheldrick GM. Crystal structure refinement withSHELXL. Acta Crystallogr C Struct Chem. 2015;71(1):3–8. doi:10.1107/s2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr. 2009;42(2):339–341. doi:10.1107/s0021889808042726
Shabunina OV, Starnovskaya ES, Shtaits YK, Kopchuk DS, Kovalev IS, Zyryanov GV, Rusinov VL, Chupakhin ON. A Modified Synthesis of 6-Aryl-3-(6-R-pyridin-2-yl)-1,2,4-triazines. Russ J Org Chem. 2018;54(10):1576–1578. doi:10.1134/s107042801810024x
Krinochkin AP, Kopchuk DS, Kozhevnikov DN. Luminescent neutral lanthanide complexes of 5-aryl-2,2′-bipyridine-6-carboxylic acids, synthesis and properties. Polyhedron. 2015;102:556–561. doi:10.1016/j.poly.2015.09.055
Moseev TD, Varaksin MV, Gorlov DA, Nikiforov EA, Kopchuk DS, Starnovskaya ES, Khasanov AF, Zyryanov GV, Charushin VN, Chupakhin ON. Direct C H/C Li coupling of 1,2,4-triazines with C6F5Li followed by aza-Diels-Alder reaction as a pot, atom, and step economy (PASE) approach towards novel fluorinated 2,2′-bipyridine fluorophores. J Fluor Chem. 2019;224:89–99. doi:10.1016/j.jfluchem.2019.05.008
Prokhorov AM., Kozhevnikov DN. 2012. Reactions of triazines and tetrazines with dienophiles (Review). Chem Heterocycl Comp. 2012;48(8):1153–1176. doi:10.1007/s10593-012-1117-9
Zhang F-G, Chen Z, Tang X, Ma J-A. Triazines: Syntheses and Inverse Electron-demand Diels–Alder Reactions. Chem Rev. 2021;121(23):14555–14593. doi:10.1021/acs.chemrev.1c00611
Chupakhin ON, Postovskii IYa. Nucleophilic Substitution of Hydrogen in Aromatic Systems. Russ Chem Rev. 1976;45(5):454-468. doi:RC1976v045n05ABEH002670
Charushin VN, Chupakhin ON. 2014. Metal Free C–H Functionalization of Aromatics, Nucleophilic Displacement of Hydrogen, in Topics in Heterocyclic Chemistry, eds. V. Charushin and O. Chupakhin, Springer. 2014;37:1–50.
Kovalev IS, Kopchuk DS, Zyryanov GV, Rusinov VL, Chupakhin ON, Charushin VN. Organolithium compounds in the nucleophilic substitution of hydrogen in arenes and hetarenes. Russ. Chem. Rev. 2015;84(12):1191-1225. doi:10.1070/RCR4462
Fatykhov RF, Savchuk MI, Starnovskaya ES, Bobkina MV, Kopchuk DS, Nosova EV, Zyryanov GV, Khalymbadzha IA, Chupakhin ON, Charushin VN, Kartsev VG. Nucleophilic substitution of hydrogen–the Boger reaction sequence as an approach towards 8-(pyridin-2-yl)coumarins. Mendeleev Commun. 2019;29(3):299-300. doi:10.1016/j.mencom.2019.05.019
Konno S, Sagi M, Takaharu E, Fujimura S, Hayashi K, Yamanaka H. Studies on as-Triazine Derivatives. XII.: Synthesis of Alkenyl-1,2,4-triazine Derivatives. Chem. Pharm. Bull. 1988;36(5):1721-1726. doi:10.1248/cpb.36.1721
Kozhevnikov DN, Kozhevnikov VN, Prokhorov AM, Ustinova MM, Rusinov VL, Chupakhin ON,Aleksandrov GG, König B. Consecutive nucleophilic substitution and aza Diels–Alder reaction—an efficient strategy to functionalized 2,2′-bipyridines. Tetrahedron Lett. 2006;47(6):869-872. doi:10.1016/j.tetlet.2005.12.006
Slovesnova NV, Minin AS, Belousova AV, Ustyugov AS, Chaprov KD, Krinochkin AP, Valieva MI, Shtaitz YaK, Starnovskaya ES, Nikonov IL, Tsmokalyuk AN, Kim GA, Santra S, Kopchuk DS, Nosova EV, Zyryanov GV. New TEMPO–Appended 2,2′–Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes: Synthesis, Photophysical Studies and Testing Photoluminescence-Based Bioimaging Abilities. Molecules. 2022;27(23):8414. doi:10.3390/molecules27238414
Kopchuk DS, Krinochkin AP, Kozhevnikov DN, Slepukhin PA. Novel neutral lanthanide complexes of 5-aryl-2,2'-bipyridine-6'-carboxylic acids with improved photophysical properties. Polyhedron. 2016;118:30–36. doi:10.1016/j.poly.2016.07.025
Halcrow MA. Jahn–Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem Soc Rev. 2013;42(4):1784–1795. doi:10.1039/c2cs35253b
Sharma Mitu, Ganeshpandian M, Majumder M, Tamilarasan A, Sharma Mukesh, Mukhopadhyay R, Islam NS, Palaniandavar M. Octahedral copper(ii)-diimine complexes of triethylenetetramine: effect of stereochemical fluxionality and ligand hydrophobicity on CuII/CuIredox, DNA binding and cleavage, cytotoxicity and apoptosis-inducing ability. Dalton Trans. 2020;49(24):8282–8297. doi:10.1039/d0dt00928h
Iqbal M, Ali S, Tahir MN. Octahedral copper(II) carboxylate complex: synthesis, structural description, DNA-binding and anti-bacterial studies. J Coord Chem. 2018;71(7):991–1002. doi:10.1080/00958972.2018.1456655
Matović ZD, Miletić VD, Ćendić M, Meetsma A, van Koningsbruggen PJ, Deeth RJ. Synthetic, crystallographic, and computational study of copper(II) complexes of ethylenediaminetetracarboxylate ligands. Inorg Chem. 2013;52(3):1238–1247. doi:10.1021/ic301609t
Vangala VR, Nangia A, Lynch VM. Interplay of phenyl–perfluorophenyl stacking, C–H⋯F, C–F⋯π and F⋯F interactions in some crystalline aromatic azinesElectronic supplementary information (ESI) available: experimental powder X-ray diffraction spectra. See http://www.rsc.org/suppdata/cc/b2/b202181a/.Chem Commun (Camb). 2002;12:1304–1305. doi:10.1039/b202181a
Bonegardt DV, Trubin SV, Sukhikh AS, Klyamer DD, Basova TV. Effect of substituent position on saturated vapor pressure of tetrafluorosubstituted zinc phthalocyanines. Žurnal neorganičeskoj himii. 2023;68(2):181–190. doi:10.31857/s0044457x22601614
Adams N, Cowley AR, Dubberley SR, Sealey AJ, Skinner MEG, Mountford PEvaluation of the relative importance of Ti–Cl⋯H–N hydrogen bonds and supramolecular interactions between perfluorophenyl rings in the crystal structures of [Ti(NR)Cl2(NHMe2)2] (R = iPr, C6H5 or C6F5)Electronic supplementary information (ESI) available: characterisation and crystal data for compounds 1–3. See http://www.rsc.org/suppdata/cc/b1/b109251k/. Chem Commun (Camb). 2001;24:2738–2739. doi:10.1039/b109251k
DOI: https://doi.org/10.15826/chimtech.2025.12.2.17
Copyright (c) 2025 Timofey D. Moseev, Mikhail V. Varaksin, Alexey P. Krinochkin, Maria A. Valieva, Ekaterina A. Kudryashova, Yulia M. Sayfutdinova, Anastasiya V. Rybakova, Dmitry S. Kopchuk, Grigory V. Zyryanov, Pavel A. Slepukhin, Vasiliy S. Gaviko, Valery N. Charushin, Oleg N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







