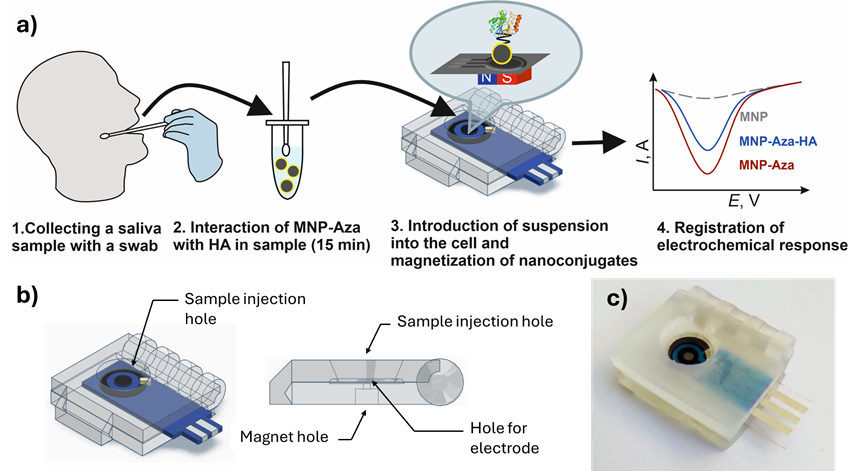
Rapid voltammetric determination of hemagglutinin in saliva using magnetic nanoparticles modified with triazolotriazine in a portable cell design
Abstract
Keywords
Full Text:
PDFReferences
Kiselev D, Matsvay A, Abramov I, Dedkov V, Shipulin G, Khafizov K. Current Trends in Diagnostics of Viral Infections of Unknown Etiology. Viruses. 2020;12(2):211. doi:10.3390/v12020211
Elaheh K.Goharshadi EK, Goharshadi K, Moghayedi M. The use of nanotechnology in the fight against viruses: A critical review. Coordinat Chem Rev. 2022;464:214559. doi:10.1016/j.ccr.2022.214559
Ozer T, Geiss BJ, Henry ChS. Review—Chemical and Biological Sensors for Viral Detection. Electrochem Soc. 2020;167(3):037523. doi:10.1149/2.0232003JES
Nirbhaya V, Chaudhary C, Chandra R, Kumar S. Biofunctionalized carbonaceous nanoflakes based efficient electrochemical biosensor for SAA biomarker detection. Appl Surf Sci Adv. 2023;13:100368. doi:10.1016/j.apsadv.2023.100368
Gish RG, Gutierrez JA, Navarro-Cazarez N, Giang K, Adler D, Tran B, Locarnini S, Hammond R, Bowden S. A simple and inexpensive point-of-care test for hepatitis B surface antigen detection: serological and molecular evaluation. J Viral Hepatitis. 2014;21(12):905–8. doi:10.1111/jvh.12257
Miyoshi-Akiyama T, Narahara K, Mori S, Kitajima H, Kase T, Morikawa S, Kirikae T. Development of an Immunochromatographic Assay Specifically Detecting Pandemic H1N1 Influenza Virus. J Clinical Microbiol. 2009;48(3):703–8. doi:10.1128/jcm.02262-09
Sakurai A, Takayama K, Nomura N, Kajiwara N, Okamatsu M, Yamamoto N, Tamura T, Yamada J. Fluorescent Immunochromatography for Rapid and Sensitive Typing of Seasonal Influenza Viruses. PLoS ONE. 2015;10(2):e0116715. doi:10.1371/journal.pone.0116715
Chen A, Chatterjee S. Nanomaterials based electrochemical sensors for biomedical applications. ChemSocRev. 2013;12:5425–38. doi:10.1039/C3CS35518G
Zhu Ch, Yang G, Li H, Du D, Lin Yu. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal Chem. 2015;87(1):230–249. doi:10.1021/ac5039863
Fablani L, Saroglia M, Fillo S, Luca V, Faggoni G, D’Amore N, Regalbuto E, Salvatori P. Magnetic based combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in salvia. Biosensors Bioelectronics. 2021;171:112686. doi:10.1016/j.bios.2020.112686
Nidzworski D, Siuzdak K, Niedziałkowski P, Bogdanowicz R, Sobaszek M, Ryl J, Weiher P, Sawczak M, Wnuk E, Goddard III WA, Jaramillo-Botero A, Ossowski T. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci Rep. 2017;7(1):15707. doi:10.1038/s41598-017-15806-7
Svalova TS, Malysheva NN, Zaidullina RA, Medvedeva MV, Mazur AV, Morshchinin IV, Kozitsina AN. Novel electrochemical immunosensing platform based on magnetite-antibody conjugate as a direct signal label: design and application for Salmonella typhimurium. Anal Lett. 2023;56(16):2572–85. doi:10.1080/00032719.2023.2180015
Singla Pr, Luxami V, Paul K. Triazine as a promising scaffold for its versatile biological behavior. Eur J Med Chem. 2015;102:39–57. doi:10.1016/j.ejmech.2015.07.037
Rusinov VL, Charushin VN, Chupakhin ON. Biologically active azolo-1,2,4-triazines and azolopyrimidines. Russ Chem Bull. 2018;67(4):573–599. doi:10.1007/s11172-018-2113-8
Ivoilova A, Mikhalchenko LV, Tsmokalyuk A, Leonova M, Lalov A, Mozharovskaia P, Kozitsina AN, Ivanova AV, Rusinov VL, Redox Conversions of 5-Methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide L-Arginine Monohydrate as a Promising Antiviral Drug. Molecules. 2021;26(16):5087. doi:10.3390/molecules26165087
Svalova TS, Medvedeva MV, Mazur AV, Drokin RA, Butorin II, Tsmokalyuk AN, Malysheva NN, Rusinov VL, Kozitsina AN. Voltammetric determination of hemagglutinin using triazolotriazine derivatives as agents for the biomolecule recognition. Electrochimica Acta. 2024;481:143954. doi:10.1016/j.electacta.2024.143954
Medvedeva MV, Mazur AV, Svalova TS, Balin IA, Rusinov VL, Matern AI, Kozitsina AN. Voltammetric determination of measles virus antibodies using a glassy carbon electrode modifed with 2-propargyltio-6-notro-7-hydroxy-4H-1,2,4-triazolo-4,7-dihydro[5,1-c]-1,2,4-triazine. J Anal Chem. 2024;78:1694–1700. doi:10.1134/S1061934823120109
Sucha L, Betteridge D, Kotrly S. Solution Equilibria in Analytical Chemistry London:Van Nostrand Reinhold Company: 1972. 371 p.
Drokin RA, Fesenko EA, Mozharovskaia PN, Medvedeva MV, Svalova TS, Kozitsina AN, EsaulkovaYaL, Volobueva AS, Zarubaev VV, Rusinov VV. 4-Hydroxy-3-nitro-1,4-dihydrotriazolo[5,1-c][1,2,4]triazines:synthesis, antiviral activity, and electrochemical characteristics. Russ Chem Bull. 2022;71(11):2460–6. doi:10.1007/s11172-022-3674-0
Liu ZL, Liu YJ, Yao KL, Ding ZH, Tao J, Wang X. Synthesis and Magnetic properties of Fe3O4. J Mater Synthesis Processing. 2002;10:83–87. doi:10.1023/A:1021231527095
Sarkar A, Xu F, Lee S. Human saliva and model saliva at bulk to adsorbed phases - similarities and differences. Adv Colloid Interface Sci. 2019;273:102034. doi:10.1016/j.cis.2019.102034
DOI: https://doi.org/10.15826/chimtech.2025.12.1.06
Copyright (c) 2024 Yulia Sabitova, Tatiana Svalova, Margarita Medvedeva, Natalia Malysheva, Vladimir Rusinov, Anatoly Matern, Alisa Kozitsina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







