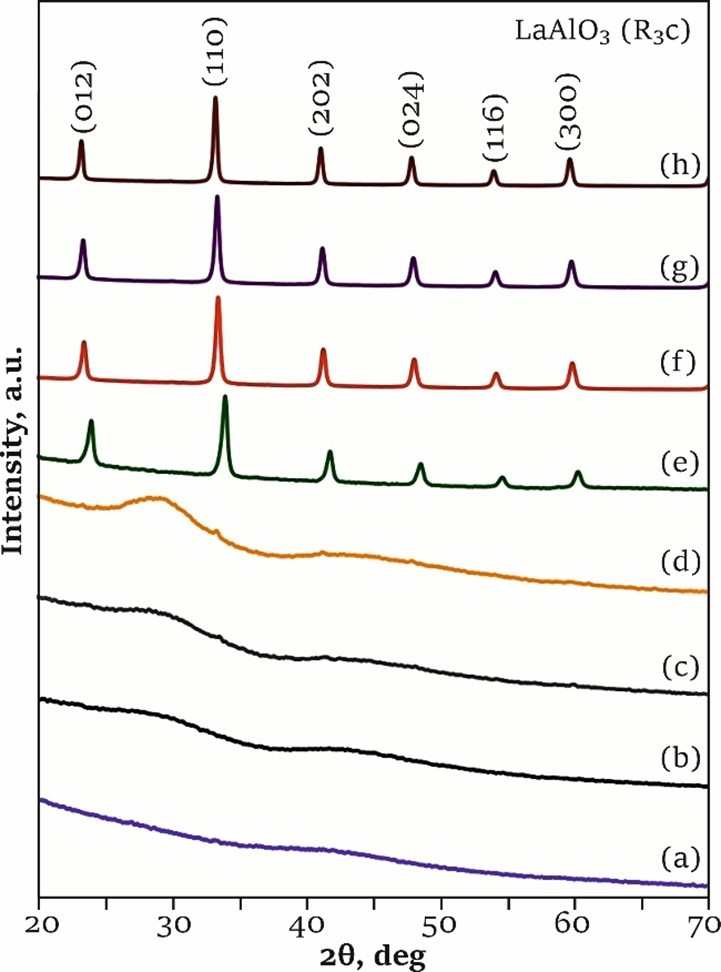
Effect of citric acid quantity on LaAlO3 formation in sol-gel synthesis with combustion
Abstract
Keywords
Full Text:
PDFReferences
Heveling J. La-Doped Alumina, Lanthanum Aluminate, Lanthanum Hexaaluminate, and Related Compounds: A Review Covering Synthesis, Structure, and Practical Importance. Ind Eng Chem Res. 2023;62:2353–86. doi:10.1021/acs.iecr.2c03007
Liu Z, Chen Q. Phase transition of LaAlO3 nanocrystal enhanced optical linear&third order nonlinear and dielectric properties of glasses. J Non-Cryst Solids. 2023;599:121965. doi:10.1016/j.jnoncrysol.2022.121965
Royer S, Duprez D, Can F, Courtois X, Batiot-Dupeyrat C, Laassiri S, Alamdari H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem Rev. 2014;114(20):10292–368. doi:10.1021/cr500032a
Chandradass J, Kim KH. Synthesis and characterization of LaAlO3 nanopowders by emulsion combustion method. J Alloys Compd. 2009;481:L31–4. doi:10.1016/j.jallcom.2009.03.072
Zhang X, Chu W, Bai H, Liang S. LaAlO3: a new high-temperature negative temperature coefficient thermistor. J Mater Sci Mater Electron. 2022;33:12093–103. doi:10.1007/s10854-022-08169-x
Vourdas N, Marathoniti E, Pandis PK, Argirusis C, Sourkouni G, Legros C, Mirza S, Stathopoulos VN. Evaluation of LaAlO3 as top coat material for thermal barrier coatings. Trans Nonferrous Met Soc China. 2018;28:1582–92. doi:10.1016/S1003-6326(18)64800-9
Lee G, Kim I, Yang I, Ha JM, Na HB, Jung JC. Effects of the preparation method on the crystallinity and catalytic activity of LaAlO3 perovskites for oxidative coupling of methane. Appl Surf Sci. 2018;429:55–61. doi:10.1016/j.apsusc.2017.08.092
Moothedan M, Sherly KB. Synthesis and characterization of nanomesoporous lanthanum aluminate (LaAlO3) for catalytic wet air oxidation of phenol. Emergent Mater. 2020;3:267–77. doi:10.1007/s42247-020-00097-y
Rivero-Antúnez P, Morales-Flórez V, Cumbrera FL, Esquivias L. Rietveld analysis and mechanical properties of in situ formed La-β-Al2O3/Al2O3 composites prepared by sol-gel method. Ceram Int. 2022;48(17):24462–70. doi:10.1016/j.ceramint.2022.05.058
Egorova AV, Belova KG, Animitsa IE, Morkhova YA, Kabanov AA. Effect of zinc doping on electrical properties of LaAlO3 perovskite. Chim Techno Acta. 2021;8(1):20218103. doi:10.15826/chimtech.2020.8.1.03
Filonova E, Medvedev D. Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomater. 2022;12(12):1991. doi:10.3390/nano12121991
Kumar P, Singh S, Gupta I, Kumar V, Singh D. Er3+ activated LaAlO3 perovskite phosphor: Crystal structure and down conversion photoluminescent behaviour for optoelectronic devices. Inorg Chem Commun. 2022;141:109578. doi:10.1016/j.inoche.2022.109578
Kumar P, Singh S, Gupta I, Kumar V, Singh D. Structural and optical characterization of trivalent samarium-activated LaAlO3 nanocrystalline materials for solid-state lighting. J Mol Struct. 2022;1265:133362. doi:10.1016/j.molstruc.2022.133362
Kumar P, Singh S, Gupta I, Kumar V, Singh D. Preparation and luminescence behaviour of perovskite LaAlO3:Tb3+ nanophosphors for innovative displays. Optik. 2022;267:169709. doi:10.1016/j.ijleo.2022.169709
Muñoz HJ, Korili SA, Gil A. Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum. Mater. 2022;15(9):3288. doi:10.3390/ma15093288
Pechini MP. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. United States patent US3330697A. 1967 Jan 7.
Negahdari Z, Saberi A, Willert-Porada M. Synthesis and characterization of nanocrystalline microwave dielectric LaAlO3 powder via sucrose method. J Alloys Compd. 2009;485:367–71. doi:10.1016/j.jallcom.2009.05.102
Zhang Q, Saito F. Mechanochemical Synthesis of Lanthanum Aluminate by Grinding Lanthanum Oxide with Transition Alumina. J Am Ceram Soc. 2000;83:439–41. doi:10.1111/j.1151-2916.2000.tb01215.x
Mizukami F, Maeda K, Watanabe M, Masuda K, Sano T, Kuno K. Preparation of Thermostable High-Surface-Area Aluminas and Properties of the Alumina-Supported Pt Catalysts. Stud Surf Sci Catal. 1991;71:557–68. doi:10.1016/S01672991(08)63002-5
Li J, Wu Y, Pan Y, Guo J. Influence of citrate-to-nitrate ratio on the thermal behavior and chemical environment of alumina gel. Ceram Int. 2007;33(5):735–8. doi:10.1016/j.ceramint.2005.12.011
Khamkar KA, Jagadale PN, Kulal SR, Bamane SR, Dhapte VV. Synthesis, characterization and electrical properties of nanostructured LaAlO3 by sol–gel auto combustion method. J Mater Sci Mater Electron. 2013;24(11):4482–7. doi:10.1007/s10854-013-1428-3
Silveira IS, Ferreira NS, Souza DN. Structural, morphological and vibrational properties of LaAlO3 nanocrystals produced by four different methods. Ceram Int. 2021;47(19):27748–58. doi:10.1016/j.ceramint.2021.06.201
Chandradass J, Balasubramanian M, Kim KH. Synthesis and Characterization of LaAlO3 Nanopowders by Various Fuels. Mater Manuf Process. 2010;25(12):1449–53. doi:10.1080/10426914.2010.508962
Belyaninova TV, Siunina LA, Mishenina LN. The sol-gel synthesis of calcium aluminate using various polymerizing agents. Tomsk State Univ J Chem. 2016;4(6):65–72. doi:10.17223/24135542/6/7
Aruna ST, Kini NS, Rajam KS. Solution combustion synthesis of CeO2–CeAlO3 nano-composites by mixture-of-fuels approach. Mater Res Bull. 2009;44(4):728–33. doi:10.1016/j.materresbull.2008.09.034
Chandradass J, Kim KH. Mixture of fuels approach for the solution combustion synthesis of LaAlO3 nanopowders. Adv Powder Technol. 2010;21:100–5. doi:10.1016/j.apt.2009.10.014
Lvov OV, Radishchevskaya NI, Nazarova AYu, Minin RV. Sintez pigmentov na osnove soyedineniy margantsa metodom goreniya rastvorov [Synthesis of pigments based on manganese compounds by combustion solutions]. In: 8th Int. Congr. Energy Fluxes Radiat. Eff. 2022 Oct 2-8; Tomsk, Russia. p. 1351–8. doi:10.56761/EFRE2022.N1-P-010801
Sakharov KA, Simonenko EP, Simonenko NP, Vaganova ML, Lebedeva YE, Chaynikova AS, Osin IV, Sorokin OYu, Grashchenkov DV, Sevastyanov VG, Kuznetsov NT, Kablov EN. Glycol-citrate synthesis of fine-grained oxides La2−xGdxZr2O7 and preparation of corresponding ceramics using FAST/SPS process. Ceram Int. 2018;44(7):7647–55. doi:10.1016/j.ceramint.2018.01.188
Jain SR, Adiga KC, Pai Verneker VR. A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust Flame. 1981;40:71–9. doi:10.1016/0010-2180(81)90111-5
Doebelin N, Kleeberg R. Profex: a graphical user interface for the Rietveld refinement program BGMN. J Appl Crystallogr. 2015;48(5):1573–80. doi:10.1107/S1600576715014685
Shakirzyanov RI, Volodina NO, Kozlovskiy AL, Zdorovets MV, Shlimas DI, Borgekov DB, Garanin YA. Study of the Structural, Electrical, and Mechanical Properties and Morphological Features of Y-Doped CeO2 Ceramics with Porous Structure. J Compos Sci. 2023;7(10):411. doi:10.3390/jcs7100411
Nath D, Singh F, Das R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater Chem Phys. 2020;239:122021. doi:10.1016/j.matchemphys.2019.122021
Roduit N. JMicroVision: Image analysis toolbox for measuring and quantifying components of high-definition images. Version 1.3.4. https://jmicrovision.github.io
Matveev AV, Nartova AV, Sankova NN, Okunev AG. DLGRAM cloud service for deep‐learning analysis of microscopy images. Microsc Res Tech. 2024;87(5):991–8. doi:10.1002/jemt.24480
Yagodina AYu, Mishenina LN. Nitrat-tsitratnyy sintez i issledovaniye svoystv lyuminoforov na osnove ortoalyuminata lantana [Nitrate-citrate synthesis and investigation of the properties of phosphors based on lanthanum orthoaluminate]. Tomsk State Univ J Chem. 2023;29:104-15. Russian. doi:10.17223/24135542/29/10
Selyunina LA, Mishenina LN, Slizhov YuG, Kozik VV. Effect of citric acid and ethylene glycol on the formation of calcium aluminate via the sol-gel method. Russ J Inorg Chem. 2013;58:450–5. doi:10.1134/S0036023613040165
Yu HF, Guo YM. Effects of heating atmosphere on formation of crystalline citrate-derived LaAlO3 nanoparticles, J Alloys Compd. 2011;509:1984–8. doi:10.1016/j.jallcom.2010.10.109
Yu HF, Wang J, Wang SS, Kuo YM. Thermochemical behavior of metallic citrate precursors for the production of pure LaAlO3. J Phys Chem Solids. 2009;70:218–23. doi:10.1016/j.jpcs.2008.10.005
Behera SK, Sahu PK, Pratihar SK, Bhattacharyya S. Phase evolution in gel-precipitated LaAlO3 ceramics. J Phys Chem Solids. 2008;69(8):2041–6. doi:10.1016/j.jpcs.2008.02.019
Mabelane TS, Koao LF, Motloung SV, Motaung TE, Kroon RE, Mhlongo MR. Effect of annealing period on the structure, morphology, and optical properties of CaAl2O4:0.1% Sm3+ prepared by citrate sol-gel method. J Mol Struct. 2022;1260:132751. doi:10.1016/j.molstruc.2022.132751
Tian ZQ, Yu HT, Wang ZL. Combustion synthesis and characterization of nanocrystalline LaAlO3 powders. Mater Chem Phys. 2007;106:126–9. doi:10.1016/j.matchemphys.2007.05.027
Tsvetkova IN, Shilova OA, Drozdova IA, Gomza YuP. Issledovaniye fraktalnoy struktury gibridnykh fosforosilikatnykh i borosilikatnykh materialov poluchennykh zol-gel metodom [Investigation of the fractal structure of hybrid phosphorosilicate and borosilicate materials obtained by the sol-gel method]. Perspektivnyye materialy. 2011;S13:888–95.
DOI: https://doi.org/10.15826/chimtech.2025.12.2.01
Copyright (c) 2024 Kristina Antropova, Ruslan Kuzmin, Natalia Aleksandrova, Alexander Yurgin, Julia Malyutina, Nomina Burkhinova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







