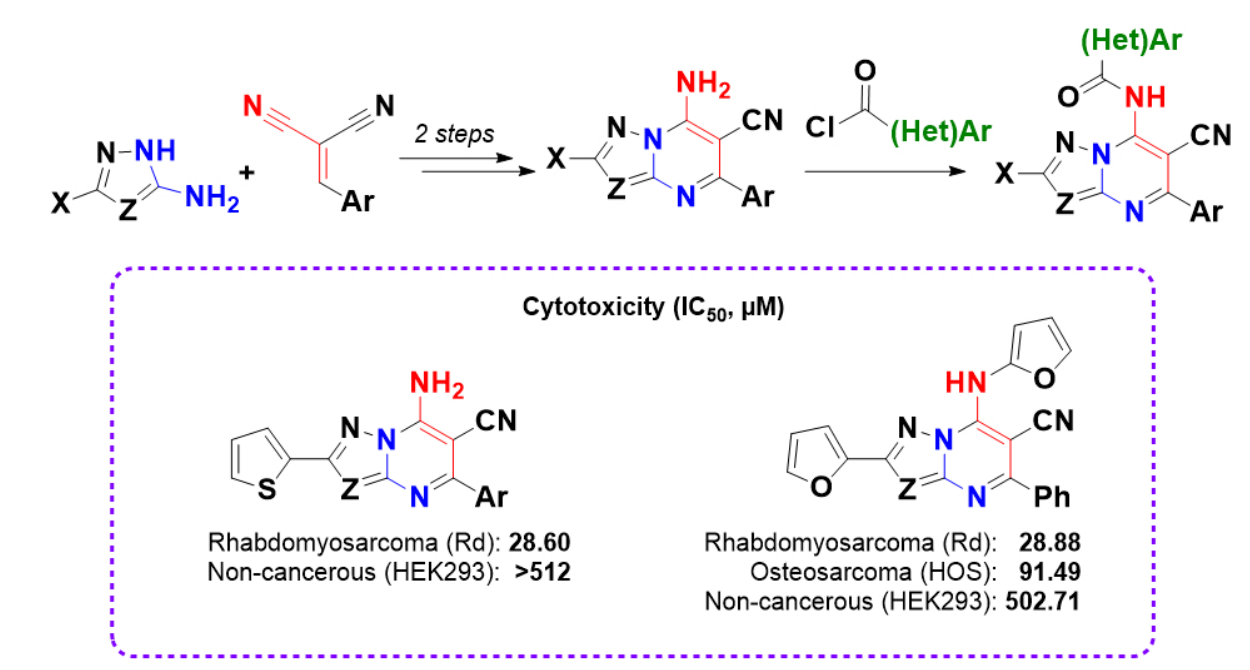
Synthesis, modification and cytotoxic properties of new 7-amino-5-arylazolo[1,5-a]pyrimidine-6-carbonitriles
Abstract
Keywords
Full Text:
PDFReferences
Saqub H, Proetsch-Gugerbauer H, Bezrookove V, Nosrati M, Vaquero EM, de Semir D, Ice RJ, McAllister S, Soroceanu L, Kashani-Sabet M, Osorio R, Dar AA. Dinaciclib, a cyclin-dependent kinase inhibitor, suppresses cholangiocarcinoma growth by targeting CDK2/5/9. Sci Rep. 2010(1);18489. doi:10.1038/s41598-020-75578-5
Franza M, Albanesi J, Mancini B, Pennisi R, Leone S, Acconcia F, Bianchi F, di Masi A. The Clinically Relevant CHK1 Inhibitor MK-8776 Induces the Degradation of the Oncogenic Protein PML-RARα and Overcomes ATRA Resistance in Acute Promyelocytic Leukemia Cells. Biochem Pharmacol. 2023;214:115675. doi:10.1016/j.bcp.2023.115675
Ito H, Nishio S, Abe M. Anagliptin in the Treatment of Type 2 Diabetes: Safety, Efficacy, and Patient Acceptability. Diabetes, Metabolic Syndrome and Obesity. Targets Therapy. 2015;163. doi:10.2147/dmso.s54679
Verster J. Clinical Evaluation of Zaleplon in the Treatment of Insomnia. Nature Sci Sleep. 2010;115. doi:10.2147/nss.s6853
Hoshiya M, Awazu M. Trapidil inhibits platelet-derived growth factor-stimulated mitogen-activated protein kinase cascade. Hypertension. 1998;31(2):665–671.
Sun Q, Liu M, Wang Q, Wang X, Lin P, Yang M, Yan Y. Effect of trapidil in myocardial ischemia-reperfusion injury in rabbit. Indian J Pharmacol, 2014;46:207. doi:10.4103/0253-7613.129320
Massari S, Bertagnin C, Pismataro MC, Donnadio A, Nannetti G, Felicetti T, Di Bona S, Nizi MG, Tensi L, Manfroni G, Loza MI, Sabatini S, Cecchetti V, Brea J, Goracci L, Loregian A, Tabarrini O. Synthesis and characterization of 1,2,4-triazolo[1,5-a]pyrimidine-2-carboxamide-based compounds targeting the PA-PB1 interface of influenza A virus polymerase. Eur J Med Chem. 2021;209. doi:10.1016/j.ejmech.2020.112944
Mayhoub AS. Hepatitis C RNA-Dependent RNA Polymerase Inhibitors: A Review of Structure–Activity and Resistance Relationships; Different Scaffolds and Mutations. Bioorganic. Med Chem. 2012;20:3150–3161. doi:10.1016/j.bmc.2012.03.049
Oukoloff K, Lucero B, Francisco KR, Brunden KR, Ballatore C. 1,2,4-Triazolo[1,5-a]pyrimidines in drug design. Eur J Med Chem. 2019;165:332–346. doi:10.1016/j.ejmech.2019.01.027
Soares de Melo C, Feng TS, van der Westhuyzen R, Gessner RK, Street LJ, Morgans GL, Warner DF, Moosa A, Naran K, Lawrence N, Boshoff HIM, Barry CE III, Harris CJ, Gordon R, Chibale K. Aminopyrazolo[1,5-a]Pyrimidines as Potential Inhibitors of Mycobacterium Tuberculosis: Structure Activity Relationships and ADME Characterization. Bioorg Med Chem. 2015;23:7240–7250. doi:10.1016/j.bmc.2015.10.021
Asati V, Anant A, Patel P, Kaur K, Gupta GD. Pyrazolopyrimidines as Anticancer Agents: A Review on Structural and Target-Based Approaches. Eur J Med Chem. 2021;225:113781. doi:10.1016/j.ejmech.2021.113781
Hammouda MM, Gaffer HE, Elattar KM. Insights into the Medicinal Chemistry of Heterocycles Integrated with a Pyrazolo[1,5-a]Pyrimidine Scaffold. RSC Med Chem. 2022;13:1150–1196. doi:10.1039/d2md00192f
Cherukupalli S, Karpoormath R, Chandrasekaran B, Hampannavar GirishA, Thapliyal N, Palakollu VNAn Insight on Synthetic and Medicinal Aspects of Pyrazolo[1,5-a]Pyrimidine Scaffold. Eur J Med Chem. 2017;126:298–352. doi:10.1016/j.ejmech.2016.11.019
Wendt MD, Kunzer A. Henry RF, Cross J, Pagano TG, Regiochemistry of addition of aminoheterocycles to α-cyanocinnamonitriles: formation of aza-bridged bi- and tricycles. Tetrahedron Lett. 2007;48:6360–6363. doi:10.1016/j.tetlet.2007.07.039
Rahmati A, One-pot synthesis of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitriles via a domino three-component condensation-oxidation reaction. Comptes Rendus Chimie. 2012;15:647–652.
Al-Mousawi SM, Moustafa MS, Elnagdi MH, Reassignment of the Structures of Products Produced by Reactions of the Product Believed To Be 2-(1-Phenyl-2-Thiocyanatoethylidene)-malononitrile with Electrophiles. Molecules. 2011;16:3456–3468. doi:10.3390/molecules16053456
Hassan AS, Mady MF, Awad HM, Hafez TS, Synthesis and antitumor activity of some new pyrazolo [1, 5-a] pyrimidines. Chin Chem Lett. 2017;28:388–393. doi:10.1016/j.cclet.2016.10.022
Said M, Eldehna W, Nocentini A, Fahim S, Bonardi A, Elgazar A, Kryštof V, Soliman D, Abdel-aziz H, Gratteri P, Abou-seri S, Supuran C. Sulfonamide-based ring-fused analogues for CAN508 as novel carbonic anhydrase inhibitors endowed with antitumor activity: Design, synthesis, and in vitro biological evaluation. Eur J Med Chem. 2020;189(2020):112019. doi:10.1016/j.ejmech.2019.112019
KJ Dechering, Timmerman M, Rensen K, J. Koolen KM, Honarnejad S, Vos MW, Huijs T, Henderson RWM, Chenu E, Laleu B, Montefiore BC, Segall MD, Mills JEJ, Guantai EM, Duffy J, Duffey M. MAIP: An Open-Source Tool to Enrich High-Throughput Screening Output and Identify Novel, Druglike Molecules with Antimalarial Activity. SLAS Discovery. 2022;27:337–348. doi:10.1021/acsmedchemlett.3c00369
Chua MM, Ortega CE, Sheikh A, Lee M, Abdul-Rassoul H, Hartshorn KL, Dominguez I. CK2 in cancer: Cellular and biochemical mechanisms and potential therapeutic target. Pharmaceuticals (Basel). 2017;10:18. doi:10.3390/ph10010018
Wińska P, Skierka, K, Łukowska-Chojnacka E, Koronkiewicz M, Cieśla J, Bretner, M. Effect of Simultaneous Inhibition of Protein Kinase CK2 and Thymidylate Synthase in Leukemia and Breast Cancer Cells. Anticancer Res. 2018;38:4617–4627. doi:10.21873/anticanres.12766
Dixit D, Sharma V, Ghosh S, Mehta VS, Sen E. Inhibition of Casein Kinase-2 Induces P53-Dependent Cell Cycle Arrest and Sensitizes Glioblastoma Cells to Tumor Necrosis Factor (TNFα)-Induced Apoptosis through SIRT1 Inhibition. Cell Death Disease. 2012;3:e271–e271. doi:10.1038/cddis.2012.10
Kim H-R, Kim K, Lee K-H, Kim SJ, Kim J. Inhibition of Casein Kinase 2 Enhances the Death Ligand- and Natural Kiler Cell-Induced Hepatocellular Carcinoma Cell Death. Clin Experim Immunol. 2008;152:336–344. doi:10.1111/j.1365-2249.2008.03622.x
Dowling JE, Chuaqui C, Pontz TW, Lyne PD, Larsen NA, Block MH, Chen H, Su N, Wu A, Russell D, Pollard H, Lee JW, Peng B, Thakur K, Ye Q, Zhang T, Brassil P, Racicot V, Bao L, Denz CR, Cooke E, Potent and Selective Inhibitors of CK2 Kinase Identified through Structure-Guided Hybridization. ACS Med Chem Lett. 2012;3:278–283. doi:10.1021/ml200257n
Sun C, Wang B, Hao S. Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Front Immunol. 2022;13:837230. doi:10.3389/fimmu.2022.837230
Launay A, Nebie O, Vijaya Shankara J, Lebouvier T, Buée L, Faivre E, Blum D, The role of adenosine A2A receptors in Alzheimer's disease and tauopathies, Neuropharmacol. 2023;226:109379. doi:10.1016/j.neuropharm.2022.109379
van der Horst E, van der Pijl R, Mulder-Krieger T, Bender A, IJzerman AP. Substructure-based virtual screening for adenosine A2A receptor ligands. ChemMedChem. 2011;6:2302–2311. doi:10.1002/cmdc.201100369
Mediavilla-Varela M, Luddy K, Noyes D, Khalil FK, Neuger AM, Soliman H, Antonia SJ. Antagonism of Adenosine A2A Receptor Expressed by Lung Adenocarcinoma Tumor Cells and Cancer Associated Fibroblasts Inhibits Their Growth. Cancer Biol Therapy. 2013;14:860–868. doi:10.4161/cbt.25643
Zhang M, Zhang A, Chen H, Chen J. Chen H, Fast Method for Synthesis of Ylidenemalononitriles. Synthetic Commun. 2006;36:3441–3445. doi:10.1080/00397910600941505
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K. and Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J Appl Crystallograph. 2009;42:339-341. doi:10.1107/S0021889808042726
G. M. Sheldrick, SHELXT – Integrated space-group and crystalstructure determination, Acta Crystallogr A Found Adv. 2015;71:3–8. doi:10.1107/S2053273314026370
Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallographica C. 2015;71:3-8. doi:10.1107/S2053229614024218
Ritz C, Baty F, Streibig JC, Gerhard D. Dose-Response Analysis Using R. PLoS One. 2015;10(12):e0146021. doi:10.1371/journal.pone.0146021
Zhang M, Zhang A, Chen H, Chen J. Chen H. Fast Method for Synthesis of Ylidenemalononitriles. Synthetic Commun. 2006;36:3441–3445. doi:10.1080/00397910600941505
DOI: https://doi.org/10.15826/chimtech.2025.12.2.02
Copyright (c) 2024 Ilya I. Butorin, Vsevolod V. Melekhin, Denis D. Chirkov, Maria D. Tokhtueva, Andrey A. Zonov, Olga A. Konovalova, Elena A. Fesenko, Anna E. Chernysheva, Pavel A. Slepukhin, Svetlana K. Kotovskaya, Vladimir L. Rusinov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







