Doping effects on the structure, transport properties, and chemical stability of LaInO3 perovskite: A review
Abstract
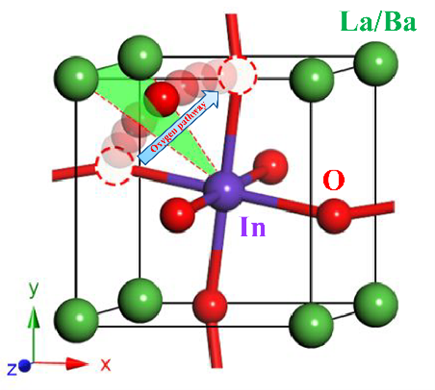
(O2–, H+) combined with high chemical stability. This review presents a comprehensive analysis of the physicochemical properties of doped LaInO3 materials. The structure and hydration processes of parent and doped compounds are discussed. The transport properties data were collected and summarized. Both the pure and the doped materials exhibit mixed ion-hole conductivity in dry air. All solid solutions based on LaInO3 are capable of reversible incorporation of water vapor due to their effective oxygen vacancy size close to ran~1.4 Å. Under elevated humidity conditions, proton transfer is observed in the samples. The data indicates a correlation between an increase in free cell volume and an increase in ionic conductivity. The results on chemical stability and TEC for the pure and doped materials are analyzed. The strategy for selecting dopant cations is shown. The presented data show the potential for applications of LaInO3-based materials in electrolyte membranes for solid oxide fuel cells, pumps and sensors.
Keywords
Full Text:
PDFReferences
Sikstrom D, Thangadurai V. A tutorial review on solid oxide fuel cells: fundamentals, materials, and applications. Ionics (Kiel). 2024. doi:10.1007/s11581-024-05824-7
Chun O, et al. Advances in low-temperature solid oxide fuel cells: An explanatory review. J Power Sources. 2024;610:234719. doi:10.1016/j.jpowsour.2024.234719
Singh M, Zappa D, Comini E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int J Hydrogen Energy. 2021;46:27643–74. doi:10.1016/j.ijhydene.2021.06.020
Qasem NAA, Abdulrahman GAQ. A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications. Int J Energy Res. 2024;2024. doi:10.1155/2024/7271748
Coduri M, et al. Structure-property correlation in oxide-ion and proton conductors for clean energy applications: Recent experimental and computational advancements. J Mater Chem A. 2022;10:5052–110. doi:10.1039/d1ta10326a
Malik V, et al. Comparative study and analysis between Solid Oxide Fuel Cells (SOFC) and Proton Exchange Membrane (PEM) fuel cell - A review. Mater Today Proc. 2021;47:2270–5. doi:10.1016/j.matpr.2021.04.203
Tariq U, et al. Bridging the Gap between fundamentals and efficient devices: Advances in proton-conducting oxides for low-temperature solid oxide fuel cells. J Power Sources. 2024;613:234910. doi:10.1016/j.jpowsour.2024.234910
Irvine J, et al. Roadmap on inorganic perovskites for energy applications. JPhys Energy. 2021;3. doi:10.1088/2515-7655/abff18
Hanif MB, et al. Mo-doped BaCe0·9Y0·1O3-δ proton-conducting electrolyte at intermediate temperature SOFCs. Part I: Microstructure and electrochemical properties. Int J Hydrogen Energy. 2023;48:37532–49. doi:10.1016/j.ijhydene.2023.01.144
Iwahara H, et al. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics. 1981;3–4:359–63. doi:10.1016/0167-2738(81)90113-2
Kreuer K-D. Proton Conductivity: Materials and Applications. Chem Mater. 1996;8:610–41. doi:10.1021/cm950192a
Chen G, et al. Ionic conduction mechanism of a nanostructured BCY electrolyte for low-temperature SOFC. Int J Hydrogen Energy. 2020;45:24108–15. doi:10.1016/j.ijhydene.2019.07.223
Matsuda RM, et al. Sintering mechanism and electrical conductivity of ZnO added BaCe0.8Zr0.1Y0.1O3-δ proton conducting perovskites. Solid State Ionics. 2023;403:116407. doi:10.1016/j.ssi.2023.116407
Soares HS, et al. Effect of the addition mechanism of ZnO sintering aid on densification, microstructure and electrical properties of Ba(Zr,Y)O3-δ proton-conducting perovskite. Int J Hydrogen Energy. 2021;46:26466–77. doi:10.1016/j.ijhydene.2021.05.109
Ebert JN, et al. Bulk and grain boundary conductivity in doped BaZrO3: Bulk contribution dominates at operating temperatures. Scr Mater. 2024;241:0–5. doi:10.1016/j.scriptamat.2023.115852
Kindelmann M, et al. Controlling grain boundary segregation to tune the conductivity of ceramic proton conductors. ChemRxiv. 2024. doi:10.26434/chemrxiv-2024-svz4w
Duan C, et al. Proton-conducting oxides for energy conversion and storage. Appl Phys Rev. 2020;7. doi:10.1063/1.5135319
Sažinas R, Bernuy-López C, Einarsrud MA, Grande T. Effect of CO2 Exposure on the Chemical Stability and Mechanical Properties of BaZrO3-Ceramics. J Am Ceram Soc. 2016;99:3685–95. doi:10.1111/jace.14395
Somekawa T, et al. Physicochemical properties of Ba(Zr,Ce)O3-Δ-based proton-conducting electrolytes for solid oxide fuel cells in terms of chemical stability and electrochemical performance. Int J Hydrogen Energy. 2017;42:16722–30. doi:10.1016/j.ijhydene.2017.04.267
Luo Y, et al. Chemical stability and electrical properties of Ba1−xCaxCe0.8Gd0.2O3−δ (0 ≤ x ≤ 0.06) proton conductor. Int J Hydrogen Energy. 2023;48:5656–67. doi:10.1016/j.ijhydene.2022.11.072
Hashim SS, et al. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int J Hydrogen Energy. 2018;43:15281–305. doi:10.1016/j.ijhydene.2018.06.045
Peng X, et al. A double perovskite decorated carbon-tolerant redox electrode for symmetrical SOFC. Int J Hydrogen Energy. 2020;45:14461–9. doi:10.1016/j.ijhydene.2020.03.151
Chen Y, et al. Advances in Cathode Materials for Solid Oxide Fuel Cells: Complex Oxides without Alkaline Earth Metal Elements. Adv Energy Mater. 2015;5:1–34. doi:10.1002/aenm.201500537
Tarutin AP, et al. Chemical design of oxygen electrodes for solid oxide electrochemical cells: A guide. Sustain Energy Technol Assessments. 2023;57:103185. doi:10.1016/j.seta.2023.103185.
Qiu P, et al. Materials of solid oxide electrolysis cells for H2 O and CO2 electrolysis: A review. J Adv Ceram. 2023;12:1463–510. doi:10.26599/JAC.2023.9220767
Li C, Soh KCK, Wu P. Formability of ABO3 perovskites. J Alloys Compd 2004;372:40–8. doi:10.1016/j.jallcom.2003.10.017
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A. 1976;32:751–67. doi:10.1107/S0567739476001551
Kasyanova AV, et al. Lanthanum-Containing Proton-Conducting Electrolytes with Perovskite Structures. Membr Membr Technol. 2021;3:73–97. doi:10.1134/S2517751621020050
Okuyama Y, et al. Incorporation and conduction of proton in Sr-doped LaMO3 (M=Al, Sc, In, Yb, Y). Electrochim Acta. 2014;125:443–9. doi:10.1016/j.electacta.2014.01.113
Lybye D, Poulsen FW, Mogensen M. Conductivity of A- and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites. Solid State Ionics. 2000;128:91–103. doi:10.1016/S0167-2738(99)00337-9
He C, Wu J, Lee Y. Correlation between conductivity and structural parameters in Sr-doped LaMO3 (M = Al, Ga, In, Er, and Y) for solid oxide membranes. Solid State Ionics. 2023;399:116315. doi:10.1016/j.ssi.2023.116315
Yu J, et al. Ionic conductivity and crystal structure of LSGM with different element mole ratios. Fuel Cells. 2021;21:149–54. doi:10.1002/fuce.202000056
Gordeev E V, Porotnikova NM. Approaches for the preparation of dense ceramics and sintering aids for Sr/Mg doped lanthanum gallate: focus review. Electrochem Mater Technol. 2023;2:20232022. doi:10.15826/elmattech.2023.2.022
Villas-Boas LA, Goulart CA, De Souza DPF. Effects of Sr and Mn co-doping on microstructural evolution and electrical properties of LaAlO3. Process Appl Ceram. 2019;13:333–41. doi:10.2298/PAC1904333V
Kalyakin A, et al. Characterization of proton-conducting electrolyte based on La0.9Sr0.1YO3–δ and its application in a hydrogen amperometric sensor. Sensors Actuators B Chem. 2016;225:446–52. doi:10.1016/j.snb.2015.11.064
Kasyanova A, et al. Thermal and Electrical Properties of Highly Dense Ceramic Materials Based on Co-doped LaYO3. Jom. 2019;71:3789–95. doi:10.1007/s11837-019-03498-5
Kasyanova AV, et al. Transport properties of LaYbO3-based electrolytes doped with alkaline earth elements. Electrochim Acta. 2023;439:141702. doi:10.1016/j.electacta.2022.141702
Kasyanova AV., et al. Low-temperature transport properties of isovalent-substituted La0.9Sr0.1YbO3–δ ceramic materials. J Solid State Electrochem. 2023. doi:10.1007/s10008-023-05574-y
Antonova EP. Proton-conducting oxides based on LaScO3: structure, properties and electrochemical applications. A focus review. Electrochem Mater Technol. 2023;2:20232021. doi:10.15826/elmattech.2023.2.021
Belova KG, et al. Conductivity and chemical stability of co-doped LaScO3 ceramics. Ceram Int. 2024;50:40321–9. doi:10.1016/j.ceramint.2024.06.148
Lesnichyova A, et al. Densification and Proton Conductivity of La1-xBaxScO3-δ; Electrolyte Membranes. Membranes (Basel). 2022;12. doi:10.3390/membranes12111084
Belyakov SA, et al. Dopant-induced changes of local structures for adjusting the hydration ability of proton-conducting lanthanum scandates. J Mater Chem A. 2023;11:19605–18. doi:10.1039/D3TA03673A
Sood K, et al. Preferential occupancy of Ca2+ dopant in La1-xCaxInO3-δ (x = 0–0.20) perovskite: structural and electrical properties. Ionics (Kiel). 2015;21:2839–50. doi:10.1007/s11581-015-1461-8
He H, Huang X, Chen L. The effects of dopant valence on the structure and electrical conductivity of LaInO3. Electrochim Acta. 2001;46:2871–7. doi:10.1016/S0013-4686(01)00508-4
Egorova A V., Belova KG, Animitsa IE. Ionic (O2−, H+) transport in novel Zn-doped perovskite LaInO3. Int J Hydrogen Energy. 2023;48:22685–97. doi:10.1016/j.ijhydene.2023.03.263
Keith ML, Roy R. Structural relations among double oxides of trivalent elements. Am Mineral J Earth Planet Mater. 1954;39:1–23.
Roth RS. Classification of Perovskite and Other ABO3-Type. J Res Natl Bur Stand. 1957;58:75. doi:10.6028/jres.058.010
Ruiz-Trejo E, Tavizón G, Arroyo-Landeros A. Structure, point defects and ion migration in LaInO3. J Phys Chem Solids. 2003;64:515–21. doi:10.1016/S0022-3697(02)00358-X
Hartley P, et al. Experimental and Theoretical Study of the Electronic Structures of Lanthanide Indium Perovskites LnInO3. J Phys Chem C. 2021;125:6387–400. doi:10.1021/acs.jpcc.0c11592
Park HM, et al. Lanthanum indium oxide from X-ray powder diffraction. Acta Crystallogr Sect C. 2003;59:i131–i132. doi:10.1107/S0108270103024806
Hu T, et al. Dy3+-doped LaInO3: a host-sensitized white luminescence phosphor with exciton-mediated energy transfer. J Mater Chem C. 2021;9:13410–9. doi:10.1039/d1tc01317c
He H, Huang X, Chen L. Sr-doped LaInO3 and its possible application in a single layer SOFC. Solid State Ionics. 2000;130:183–93. doi:10.1016/S0167-2738(00)00666-4
Srivastava AM, et al. Spectroscopy of Mn4+ in orthorhombic perovskite, LaInO3. J Lumin. 2019;206:398–402. doi:10.1016/j.jlumin.2018.10.090
Hwang KJ, et al. Molecular dynamics simulation of oxygen ion conduction in orthorhombic perovskite Ba-doped LaInO3 using cubic and orthorhombic model. J Nanosci Nanotechnol. 2015;15:8947–50. doi:10.1166/jnn.2015.11540
Yukhno E, et al. Excitation and emission spectra of LaInO3-based solid solutions doped with Sm3+, Sb3+. J Lumin. 2017;182:123–9. doi:10.1016/j.jlumin.2016.10.020
Kamal CS, et al. Unravelling the energy transfer mechanism in bismuth co-activation of LaInO3:Sm3+/Ho3+ nanophosphor for color-tunable luminescence. RSC Adv. 2017;7:9724–31. doi:10.1039/C6RA28719K
Yukhno EK, et al. Magnetic properties of LaInO3-based perovskite-structure photoluminescent materials doped with Nd3+, Cr3+, and Mn3+ ions. Inorg Mater. 2016;52:218–24. doi:10.1134/S0020168516010155
Hu X, Piccinelli F, Bettinelli M. White light emission and energy transfer processes in LaInO3 doped with Bi3+, Tb3+ and Eu3+. J Alloys Compd. 2022;899:163344. doi:10.1016/j.jallcom.2021.163344
Lakshminarasimhan N, Varadaraju UV. Luminescent host lattices, LaInO3 and LaGaO3—A reinvestigation of luminescence of d10 metal ions. Mater Res Bull. 2006;41:724–31. doi:10.1016/j.materresbull.2005.10.010
Dhanasekaran P, Gupta NM. Effects of grain morphology, microstructure and dispersed metal cocatalyst on the photoreduction of water over impurity-doped LaInO3. Mater Res Bull. 2012;47:1217–28. doi:10.1016/j.materresbull.2012.01.031
Nomura K, Tanase S. Electrical conduction behavior in (La0.9Sr0.1)MIIIO3-δ (MIII = Al, Ga, Sc, In, and Lu) perovskites. Solid State Ionics. 1997;98:229–36. doi:10.1016/s0167-2738(97)00101-x
Cook RL, Sammells AF. On the systematic selection of perovskite solid electrolytes for intermediate temperature fuel cells. Solid State Ionics. 1991;45:311–21. doi:10.1016/0167-2738(91)90167-A
Sammells AF, et al. Rational selection of advanced solid electrolytes for intermediate temperature fuel cells. Solid State Ionics. 1992;52:111–23. doi:10.1016/0167-2738(92)90097-9
Ranløv J, et al. Criteria for prediction of high oxide ion conductivity in perovskite oxides. Solid State Phenom. 1994;39:219–22.
Levy MR, Steel BCH, Grimes RW. Divalent cation solution in A3+B3+O3 perovskites. Solid State Ionics. 2004;175:349–52. doi:10.1016/j.ssi.2004.02.072
Sood K, Singh K, Pandey OP. Co-existence of cubic and orthorhombic phases in Ba-doped LaInO3 and their effect on conductivity. Phys B Condens Matter. 2015;456:250–7. doi:10.1016/j.physb.2014.08.036
Sood K, Singh K, Pandey OP. Study of the Structural and Electrical Behaviour of Ca Doped LaInO3 Electrolyte Material. Trans Indian Ceram Soc. 2013;72:32–5. doi:10.1080/0371750X.2013.795728
He HP, Huang XJ, Chen LQ. Effective way to detect the secondary phase in Sr-doped LaInO3. J Phys Chem Solids. 2001;62:701–9. doi:10.1016/S0022-3697(00)00220-1
Sood K, Singh K, Pandey OP. Studies on Sr substituted lanthanum indate as mixed ionic conductor. J Mater Sci. 2012;47:4520–9. doi:10.1007/s10853-012-6297-2
Kim HL, et al. Electrical conduction behavior of BaO-doped LaInO3 perovskite oxide. Japanese J Appl Physics. 2006;45:872–4. doi:10.1143/JJAP.45.872
Yoon M-Y, et al. Molecular dynamics simulation of the effect of dopant distribution homogeneity on the oxide ion conductivity of Ba-doped LaInO3. J Power Sources. 2014;248:1085–9. doi:10.1016/j.jpowsour.2013.10.054
Byeon D-S, et al. Oxide ion diffusion in Ba-doped LaInO3 perovskite: A molecular dynamics study. J Power Sources. 2013;222:282–7. doi:10.1016/j.jpowsour.2012.08.091
Kim H-L, et al. Oxygen ion conduction in barium doped LaInO3 perovskite oxides. J Power Sources. 2014;267:723–30. doi:10.1016/j.jpowsour.2014.06.006
Kim H-L, Kim S, Lee H-L. Phase Formation and Proton Conduction of La0.6Ba0.4In1-yMyO3-δ (M= Ga3+, Sc3+, Yb3+) System. J Korean Ceram Soc. 2002;39:610–5. doi:10.4191/kcers.2002.39.6.610
Hwang K-J, et al. The Effect of Co-Doping at the A-Site on the Structure and Oxide Ion Conductivity in (Ba0.5−xSrx)La0.5InO3−δ: A Molecular Dynamics Study. Materials (Basel). 2019;12. doi:10.3390/ma12223739
Lee KH, et al. Phase formation and electrical conductivity of Ba-doped LaScO3. Japanese J Appl Physics. 2005;44:5025–9. doi:10.1143/JJAP.44.5025
Kim S, Lee KH, Lee HL. Proton conduction in La0.6Ba0.4ScO2.8 cubic perovskite. Solid State Ionics. 2001;144:109–15. doi:10.1016/S0167-2738(01)00887-6
Kato H, et al. Electrical conductivity of Al-doped La1-xSrxScO3 perovskite-type oxides as electrolyte materials for low-temperature SOFC. Solid State Ionics. 2003;159:217–22. doi:10.1016/S0167-2738(03)00101-2
Kumar S, et al. Study of structural, dielectric, optical properties and electronic structure of Cr-doped LaInO3 perovskite nanoparticles. Mater Charact. 2017;131:108–15. doi:10.1016/j.matchar.2017.07.001
Nishiyama S, Kimura M, Hattori T. P-Type Electrical Conduction of LaInO3 Based Ceramics and Calculation of its Density of States. Key Eng Mater. 2001;216:65–8. doi:10.4028/www.scientific.net/KEM.216.65
Egorova AV, et al. Oxygen ionic transport in LaInO3 and LaIn0.5Zn0.5O2.75 perovskites: Theory and experiment. Solid State Ionics. 2021;372:115790. doi:10.1016/j.ssi.2021.115790
Belova KG, et al. Electrical Properties of Co-doped LaInO3 Perovskite. Russ J Inorg Chem. 2024. doi:10.1134/S0036023623602763
Thangadurai V, Weppner W. Synthesis and Electrical Properties of K- and Pr-Substituted LaGaO3 and LaInO3 Perovskites. J Electrochem Soc. 2001;148:A1294. doi:10.1149/1.1414286
Okuyama Y, et al. Proton Conduction and Incorporation into La1−xBaxYb0.5In0.5O3−δ. Mater Trans. 2018;59:14–8. doi:10.2320/matertrans.MB201701
Nomura K, et al. Proton conduction in (La0.9Sr0.1)MIIIO3-δ (MIII=Sc, In, and Lu) perovskites. Solid State Ionics. 2002;154–155:647–52. doi:10.1016/S0167-2738(02)00512-X
Animitsa I, et al. Incorporation of water in strontium tantalates with perovskite-related structure. Solid State Ionics. 2001;145:357–64. doi:10.1016/S0167-2738(01)00931-6
Uritsky MZ, Tsidilkovski VI. Proton transport in doped yttrium oxide. Monte-Carlo simulation. Russ J Electrochem. 2012;48:917–21. doi:10.1134/S1023193512090145
Putilov LP, Tsidilkovski VI. Impact of bound ionic defects on the hydration of acceptor-doped proton-conducting perovskites. Phys Chem Chem Phys. 2019;21:6391–406. doi:C8CP07745B
Hong SJ, Virkar A V. Lattice Parameters and Densities of Rare-Earth Oxide Doped Ceria Electrolytes. J Am Ceram Soc. 1995;78:433–9. doi:10.1111/j.1151-2916.1995.tb08820.x
Marrocchelli D, et al. Understanding Chemical Expansion in Non-Stoichiometric Oxides: Ceria and Zirconia Case Studies. Adv Funct Mater. 2012;22:1958–65. doi:10.1002/adfm.201102648
Hirata Y, et al. Synthesis and electrical conductivity of (La1−xSrx)(Al1−yMgy)O3−δ perovskite solid solution. J Asian Ceram Soc. 2014;2:176–84. doi:10.1016/j.jascer.2014.03.005
Chatzichristodoulou C, et al. Size of oxide vacancies in fluorite and perovskite structured oxides. J Electroceramics. 2015;34:100–7. doi:10.1007/s10832-014-9916-2
Marrocchelli D, Perry NH, Bishop SR. Understanding chemical expansion in perovskite-structured oxides. Phys Chem Chem Phys. 2015;17:10028–39. doi:10.1039/C4CP05885B
Zhang J, et al. Tuning oxygen vacancies in epitaxial LaInO3 films for ultraviolet photodetection. Opt Lett. 2022;47:5044. doi:10.1364/ol.470587
Rogers DB, Honig JM, Goodenough JB. The electrical properties and band structure of doped LaInO3. Mater Res Bull. 1967;2:223–30. doi:10.1016/0025-5408(67)90061-X
Galazka Z, et al. Melt Growth and Physical Properties of Bulk LaInO3 Single Crystals. Phys Status Solidi. 2021;218:2100016. doi:10.1002/pssa.202100016
Jang DH, et al. Single crystal growth and optical properties of a transparent perovskite oxide LaInO3. J Appl Phys. 2017;121:125109. doi:10.1063/1.4977863
Kilner JA, Brook RJ. A study of oxygen ion conductivity in doped non-stoichiometric oxides. Solid State Ionics. 1982;6:237–52. doi:10.1016/0167-2738(82)90045-5
Zheng Y-S, et al. Electronic Origin of Oxygen Transport Behavior in La-Based Perovskites: A Density Functional Theory Study. J Phys Chem C. 2019;123:275–90. doi:10.1021/acs.jpcc.8b11249
Egorova AV., et al. Ionic (O2– and H+) Transport in Oxygen-Deficient Perovskites La2Me+3ZnO5.5. Russ J Electrochem. 2023;59:276–83. doi:S1023193523040055
Codorniu-Hernández E, Kusalik PG. Probing the mechanisms of proton transfer in liquid water. Proc Natl Acad Sci. 2013;110:13697–8. doi:10.1073/pnas.1312350110
Pauliukaite R, Juodkazytė J, Ramanauskas R. Theodor von Grotthuss’ Contribution to Electrochemistry. Electrochim Acta. 2017;236:28–32. doi:10.1016/j.electacta.2017.03.128
Mogensen M, et al. Factors controlling the oxide ion conductivity of fluorite and perovskite structured oxides. Solid State Ionics. 2004;174:279–86. doi:10.1016/j.ssi.2004.07.036
Zvonareva IA, Medvedev DA. Proton-conducting barium stannate for high-temperature purposes: A brief review. J Eur Ceram Soc. 2023;43:198–207. doi:10.1016/j.jeurceramsoc.2022.10.049
Lesnichyova AS, et al. Proton conductivity and mobility in Sr-doped LaScO3 perovskites. Ceram Int. 2021;47:6105–13. doi:10.1016/j.ceramint.2020.10.189
Okuyama Y, et al. Proton transport properties of La0.9M0.1YbO3−δ (M=Ba, Sr, Ca, Mg). Electrochim Acta. 2013;95:54–9. doi:10.1016/j.electacta.2013.01.156
Nguyen TL, et al. Hashimoto T. The effect of oxygen vacancy on the oxide ion mobility in LaAlO3-based oxides. Solid State Ionics. 2000;130:229–41. doi:10.1016/S0167-2738(00)00640-8
Lerch M, Boysen H, Hansen T. High-temperature neutron scattering investigation of pure and doped lanthanum gallate. J Phys Chem Solids. 2001;62:445–55. doi:10.1016/S0022-3697(00)00078-0
Nomura K, Kageyama H. Neutron diffraction study of LaScO3-based proton conductor. Solid State Ionics. 2014;262:841–4. doi:10.1016/j.ssi.2013.09.018
Stroeva AY, et al. Effect of scandium sublattice defectiveness on ion and hole transfer in LaScO3-based proton-conducting oxides. Russ J Electrochem. 2011;47:264–74. doi:10.1134/S102319351103013X
Stroeva AY, et al. Phase composition and conductivity of La1-xSrxScO3-α (x = 0.01-0.20) Under oxidative conditions. Russ J Electrochem. 2012;48:509–17. doi:10.1134/S1023193512050114
Farlenkov AS, et al. Local disorder and water uptake in La1–xSrxScO3–δ. Solid State Ionics. 2017;306:82–8. doi:10.1016/j.ssi.2017.04.018
Yukhno EK, et al. Physical and chemical properties of LaInO3-based phosphors doped with Dy3+, Ho3+, Sb3+ ions. Proceedings of the National Academy of Sciences of Belarus, Chemical series. 2017;4:31–7. Russian.
Inaba H, Hayashi H, Suzuki M. Structural phase transition of perovskite oxides LaMO3 and La0.9Sr0.1MO3 with different size of B-site ions. Solid State Ionics. 2001;144:99–108. doi:10.1016/S0167-2738(01)00904-3
da Silva CA, de Miranda PE V. Synthesis of LaAlO3 based materials for potential use as methane-fueled solid oxide fuel cell anodes. Int J Hydrogen Energy. 2015;40:10002–15. doi:10.1016/j.ijhydene.2015.06.019
Lesnichyova A, et al. Water uptake and transport properties of La1-xCaxScO3-α proton-conducting oxides. Mater (Basel). 2019;12. doi:10.3390/ma12142219
Liu L. et al. Preparation, sinterability, electrical transport and thermal expansion of perovskite-type La0.8Ca0.2CrO3 composites. Appl Sci. 2020;10:4634. doi:10.3390/app10134634
Tietz F. Thermal expansion of SOFC materials. Ionics (Kiel). 1999;5:129–39. doi:10.1007/BF02375916
DOI: https://doi.org/10.15826/chimtech.2025.12.1.11
Copyright (c) 2024 Anastasia Egorova, Ksenia Belova, Irina Animitsa

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







