Synthesis and luminescence of 3-(pyridine-2-yl)-1,2,4-triazine-based Ir(III) complexes
Abstract
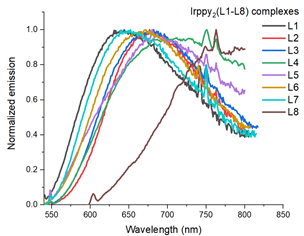
A series of novel iridium(III) complexes containing 5-N-(aryl)-amino- or 5-cycloamino-3-(pyridine-2-yl)-1,2,4-triazine ligands was obtained. These complexes exhibited red luminescence in solution as well as in the solid state. Based on the DFT studies it was suggested that N(2) atom of the 1,2,4-triazine core is preferable to N(4) one as the coordination site in the complexes of Ir(III).
Keywords
Full Text:
PDFReferences
Zheg D, Yuan X-A, Liu J-C, Li L, Wang L-P, Qin M-F, Bao S-S, Ma J, Zheng L-M. Cyclometalated Iridium(III) Complexes Incorporating Aromatic Phosphonate Ligands: Syntheses, Structures, and Tunable Optical Properties. ACS Omega. 2019;4:16543. doi:10.1021/acsomega.9b02311
Zhou J, Li J, Zhang KY, Liu S, Zhao Q. Phosphorescent iridium(III) complexes as lifetime-based biological sensors for photoluminescence lifetime imaging microscopy. Coord Chem Rev. 2022;453:21433. doi:10.1016/j.ccr.2021.214334
Zysman-Colman E. Iridium(III) in Optoelectronic and Photonics Applications. Wiley. Hoboken. 2017. doi:10.1002/9781119007166
Jayabharathy J, Thanikachalam V., Thilagavathy S. Phosphorescent organic light-emitting devices: Iridium based emitter materials – An overview. Coord Chem Rev. 2023;483:215100. doi:10.1016/j.ccr.2023.215100
Housecroft CE, Constable EC. Over the LEC rainbow: Colour and stability tuning of cyclometalated iridium(III) complexes in light-emitting electrochemical cells. Coord Chem Rev. 2017;350:155. doi:10.1016/j.ccr.2017.06.016
Vásquez B, Bayas M, Dreyse P, Palma JL, Cabrera AR, Rossin E, Natali M, Saldias C, González-Pavez I. Synthesis and Characterization of Iridium(III) Complexes with Substituted Phenylimidazo(4,5-f)1,10-phenanthroline Ancillary Ligands and Their Application in LEC Devices. Molecules. 2024;29:53. doi:10.3390/molecules29010053
Bell JD, Murphy JA. Recent advances in visible light-activated radical coupling reactions triggered by (I) ruthenium, (II) iridium and (III) organic photoredox agents. 2021;50:9540. doi:10.1039/D1CS00311A
Zakis JM, Messinis AM, Ackermann L, Smejkal T, Wencel Delord J. Air-stable bis-cyclometalated iridium catalysts for ortho-directed C(sp2)–H borylation. Adv Synth Cat. 2024;366:2292. doi:10.1002/adsc.202301411
Geraci A, Stojiljković U, Antien K, Salameh N, Baudoin O. Iridium(III)-Catalyzed Intermolecular C(sp3)-H Amidation for the Synthesis of Chiral 1,2-Diamines. Angew Chem Int Ed. 2023;62:e202309263. doi:10.1002/anie.202309263
Kamada K, Jung J, Wakabayashi T, Sekizawa K, Sato S, Morikawa T, Fukuzumi S, Saito S. Photocatalytic CO2 Reduction Using a Robust Multifunctional Iridium Complex toward the Selective Formation of Formic Acid. JACS. 2020;142:10261. doi:10.1021/jacs.0c03097
Gärtner F, Cozzula D, Losse S, Boddien A, Anilkumar G, Junge H, Schulz T, Marquet N, Spannenberg A, Gladiali S. Beller M. Synthesis, Characterization and Application of Iridium(III) Photosensitisers for Catalytic Water Reduction. Chem Eur J. 2011;17:6998. doi:10.1002/chem.201100235
Yang T, Zhu M, Jiang M, Yang F and Zhang Z. Current status of iridium-based complexes against lung cancer. Front Pharmacol. 2022;13:1025544. doi:10.3389/fphar.2022.1025544
S SA, P S, Roy N, Paira P. Advances in novel iridium(III) based complexes for anticancer applications: A review. Inorg Chim Acta. 2022;513:119925. doi:10.1016/j.ica.2020.119925
Ma D-L, Wu C, Wu K-J, Leung C-H. Iridium (III) complexes targeting Apoptoic cell death in cancer cells. 2019;24:2739. doi:10.3390/molecules24152739
Zhou L, Li J, Chen J. Yao X, Zeng X, Liu Y, Wang Y. Wang X. Anticancer activity and mechanism studies of photoactivated iridium (III) complexes toward lung cancer A549 cells. Dalton Trans. 2024;53:15176. doi:10.1039/D4DT01677G
Huang C, Liang C, Sadhukhan T, Banerjee S, Fan Z, Li T, Zhu Z, Zhang P, Raghavachari K, Huang H. In-vitro and In-vivo Photocatalytic Cancer Therapy with Biocompatible Iridium(III) Photocatalysts Angew. Chem Int Ed. 2021;60:9474. doi:10.1002/ange.202015671
Chen J, Guo X, Li D, Tang H, Gao J, Yu W, Zhu X, Sun Z, Huang Z, Chen L. Mitochondria-targeted cyclometalated iridium-β-carboline complexes as potent non-small cell lung cancer therapeutic agents. Metallomics. 2023;15:mfad035. doi:10.1093/mtomcs/mfad035
Guan R, Xie L, Ji L, Chao H. Phosphorescent Iridium(III) Complexes for Anticancer Applications. Eur J Inorg Chem. 2020;42:3978. doi:10.1002/ejic.202000754
Das R, Das U, Roy N, Mukherjee C, U S, Paira P. A glance on target specific PDT active cyclometalated iridium complexes. Dyes Pigm. 2024;226:112134. doi:10.1016/j.dyepig.2024.112134
Zhang H, Chen X, Li S, Shen J, Mao Z-W. An Enhanced Photothermal Therapeutic Iridium Hybrid Platform Reversing the Tumor Hypoxic Microenvironment. Molecules. 2022;27:2629. doi:10.3390/molecules27092629
Tang S-J, Li Q-F, Wang M-F, Yang R, Zeng L-Z, Li X-L, Wang R-D, Zhang H, Ren X, Zhang D, Gao F. Bleeding the Excited State Energy to the Utmost: Single-Molecule Iridium Complexes for In Vivo Dual Photodynamic and Photothermal Therapy by an Infrared Low-Power Laser. Adv Healthcare Mater. 2023:12:2301227. doi:10.1002/adhm.202301227
Wu N, Cao J-J, Wu X-W, Tan C-P, Ji L-N, Mao Z-W. Iridium(III) complexes with five-membered heterocyclic ligands for combined photodynamic therapy and photoactivated chemotherapy. Dalton Trans. 2017;46:13482. doi:10.1039/C7DT02477K
Joshi B, Shivashankar M. Recent advancement in the synthesis of Ir-based complexes. ACS Omega. 2023:8:43408. doi:10.1021/acsomega.3c04867
Hasan K, Bansal A, Samuel I, Roldan-Carmona C, Bolink H, Zysman-Colman E. Tuning the Emission of Cationic Iridium (III) Complexes Towards the Red Through Methoxy Substitution of the Cyclometalating Ligand. Sci Rep. 2015;5:12325. doi:10.1038/srep12325
Jing S, Wu X, Niu D, Wang J, Leung C-H, Wang W. Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy. Molecules. 2024;29:256. doi:10.3390/molecules29010256
Zhang Y, Qiao J. Near-infrared emitting iridium complexes: Molecular design, photophysical properties, and related applications. iScience. 2021;24:102858. doi:10.1016/j.isci.2021.102858
Huang C, Ran G, Zhao Y, Wang C, Song Q. Synthesis and application of a water-soluble phosphorescent iridium complex as turn-on sensing material for human serum albumin. Dalton Trans. 2018;47:2330. doi:10.1039/c7dt04676f
Xie L, Shi L, Xiong K, Guan R, Chen Ym Long J, Ji L, Chao H. Synthesis, subcellular localization and anticancer mechanism studies of unsymmetrical iridium (III) complexes. Eur J Inorg Chem. 2023;26:e202300001. doi:10.1002/ejic.202300001
Kozhevnikov VN, Deary M, Mantso T, Panayiotidis M, Sims M. Iridium(III) complexes of 1,2,4-triazines as potential bioorthogonal reagents: metal coordination facilitates luminogenic reaction with strained cyclooctynes. Chem Commun. 2019:55:14283. doi:10.1039/C9CC06828G
Kozhevnikov VN, Kozhevnikov DN, Nikitina TV, Rusinov VL, Chupakhin ON, Zabel M, Konig B. A versatile strategy for the synthesis of functionalized 2,2’-bi- and 2,2’:6’,2’-terpyridines via their 1,2,4-triazine analogues. J Org Chem. 2003;68:2882. doi:10.1021/jo0267955
Kopchuk DS, Chepchugov NV, Kovalev IS, Santra S, Rahman M, Giri K, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON. Solvent-free synthesis of 5-(aryl/alkyl)amino-1,2,4-triazines and α-arylamino-2,2’-bipyridines with greener prospects. RSC Adv. 2017;7:9610. doi:10.1039/C6RA26305D
Starnovskaya ES, Shtaitz YK, Krinochkin AP, Khasanov AF, Kopchuk DS, Zyryanov GV, Rusinov VL, Chupakhin ON. The synthesis of 6-phenoxyphenylamino-2,2’-bipyridines as new fluorophores. AIP Conf Proc. 2019;2063:040056. doi:10.1063/1.5087388
Guda MR, Valieva MI, Kopchuk DS, Aluru R, Khasanov AF, Taniya OS, Novikov AS, Zyryanov GV, Ranu BC. One-pot Synthesis and Photophysical Studies of Α-cycloamino-substituted 5-aryl-2,2'-bipyridines. J Fluoresc. 2024;34:579. doi:10.1007/s10895-023-03304-1
Porrès L, Holland A, Pålsson LO, Monkman AP, Kemp C, Beeby A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J Fluoresc. 2006:16:267. doi:10.1007/s10895-005-0054-8
Neese F, Wennmohs F, Becker U, Riplinger C. The ORCA quantum chemistry program package. J Chem Phys. 2020;152:224108. doi:10.1063/5.0004608
DOI: https://doi.org/10.15826/chimtech.2025.12.1.09
Copyright (c) 2024 Basim S. M. Al-Ghezi, Igor S. Kovalev, Maria V. Sangalova, Alena A. Noskova, Albert F. Khasanov, Nikita S. Glebov, Yaroslav K. Shtaitz, Maria I. Valieva, Alexey P. Krinochkin, Olga V. Shabunina, Dmitry S. Kopchuk, Grigory V. Zyryanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







