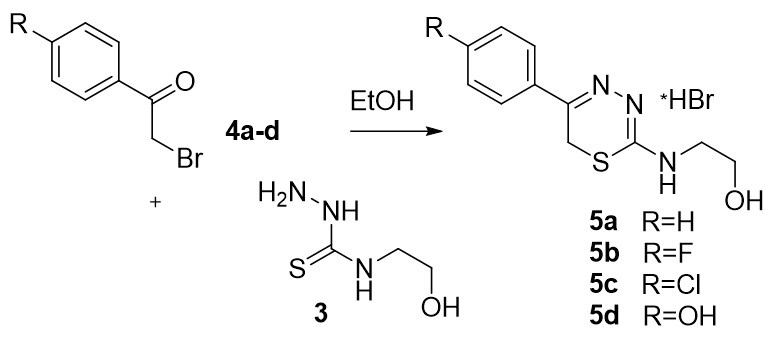
Optimal strategy of new 6H-1,3,4-thiadiazines synthesis in search of the promising antidiabetic agents
Abstract
Keywords
Full Text:
PDFReferences
Karade NN. 9.08 - 1,3,4-Oxadiazines and 1,3,4-Thiadiazines Comprehensive Heterocyclic Chemistry IV. University of Greifswald, Greifswald, Germany. 2022;9:402–455. doi:10.1016/B978-0-12-818655-8.00094-9
Meshram P, Pardhi M, Bamgude P, Kamble D, Chavan P, Tupare S. Review on Synthetic Strategies for 1,3,4-Thiadiazines and its Biological Activity. Biointerface Res Appl Chem. 2024;14(4):46. doi:10.33263/BRIAC142.046
Pfeiffer W-D. 9.08 - 1,3,4-Oxadiazines and 1,3,4-Thiadiazines Comprehensive Heterocyclic Chemistry III. University of Greifswald, Greifswald, Germany. 2008; 9:401-455. doi:10.1016/B978-008044992-0.00808-7
Chupakhin ON, Sidorova LP, Tarakhty EA, Novikova AP, Perova NM, Vinogradov VA, Van Ginkel MF. Substituted 6-H-1,3,4-thiadiazine-2-amines, the use thereof as anaesthetizing, cardiovascular and hypometabolic agents, and a pharmaceutical composition containing them United States patent US-6028068-A. 1995. Dec 28
Morvan M, Nadler G, Zimmermann RG. ChemInform Abstract:Synthesis of 1,3,4-Thiadiazin-2-one and 1,3,4-Selenadiazin-2-one Derivatives as New Cardiotonic Drugs. ChemInform. 2010;23(4):238–238. doi:10.1002/chin.199204238
Sugawara H, Endoh M. (-)-Enantiomer EMD 57439 antagonizes the Ca2+ sensitizing effect of (+)-enantiomer EMD 57033 on diastolic function but not on systolic function in rabbit ventricular cardiomyocytes. Jpn J Pharmacol. 1999;80(1):55–65. doi:10.1254/jjp.80.55
Schröder J, Henke A. Structure-Based Design and Synthesis of Potent Matrix Metalloproteinase Inhibitors Derived from a 6H-1,3,4-Thiadiazine Scaffold. J Med Chem. 2001;44(20):3231–3243. doi:10.1021/jm010887p
Ajeet A, Tripathi L, Kumar P. Designing of Novel 6(H)-1,3,4-Thiadiazine Derivatives as MMP12 Inhibitors: A MLR and Docking Approach. AJPS. 2013;1(2):29–34. doi:10.12691/ajps-1-2-3
Dhongade SR, Kenawade SA. An efficient, greener microwave assisted multi-component one pot synthesis of [1,3,4] thiadiazin-2-yl-thiazolidin-4-ones. Int J Pharm Sci Res. 2018;9(2):650–655 doi:10.13040/IJPSR.0975-8232.9(2).650-55
Rasina LN, Chupakhin ON. The estimation of the perspectives of the 1,3,4-thiadiazine derivatives as the protectors means for the health tissues under radiation therapy of malignant tumour patients. Radiats Biol Radioecol [Internet]. 2005;45(6):675–679. Russian. Available from: PubMed PMID: 16454334.
Gerasimova EL, Gazizullina EG, Igdisanova DI, Sidorova LP, Tseitler TA, Emelianov VV, Chupakhin ON, Ivanova AV. Antioxidant properties of 2,5-substituted 6H-1,3,4-thiadiazines promising for experimental therapy of diabetes mellitus. Bull Acad Sci USSR div Chem Sci. 2022;71(12):2730–2739 doi:10.1007/s11172-022-3702-0
Verma PK. Synthesis, Antimicrobial and Antioxidant Evaluation of Novel 5,6-dihydro3-(substituted phenyl)[1,3,4]thiadiazine-7-one Derivatives. Med Chem. 2017;7(5):880–883. doi:10.4172/2161-0444.1000446
Tseitler TA, Sidorova LP, Emel'yanov VV, Savateeva EA, Chupakhin ON. Synthesis and Antiglycating Activity of 2-Aminopropylmorpholino-5-Aryl-6H-1,3,4-Thiadiazine Dihydrobromides and 2-Aminopropylmopholino-5-Thienyl-6H-1,3,4-Thiadiazine Dihydrobromides Pharm Chem J. 2020;53:890–894. doi:10.1007/s11094-020-02095-0
Emelianov VV, Gette IF, Danilova IG, Sidorova LP, Tseiler TA, Mukhlynina EA. Impact of a 1,3,4-Thiadiazine Compound on Oxidative Stress Intensity in Experimental Type 2 Diabetes Mellitus. Pharm Chem J. 2023;57:353–357. doi:10.1007/s11094-023-02889-y
Beyer H. Neuere Ergebnisse über 1,3,4‐Thiadiazine. Z Chem. 1969;9(10):361-369. doi:10.1002/zfch.19690091002
Usol'tseva SV, Andronnikova GP. 1,3,4-Thiadiazines:Methods of synthesis and reactivity (review). Chem Heterocycl Compd. 1991;27(4):343–354. doi:10.1002/chin.199238326
Khidre RE, Abdel-Wahab BF, Awad GEA. A Multi-Component One-Pot Synthesis of Novel (1,3,4-Thiadiazin-2-ylamino)isoindoline-1,3-diones as Antimicrobial Agents. Heterocycles. 2017;94(2):314-325. doi:10.3987/COM-16-13611
Moghimi S, Shiri M, Heravi MM, Kruger HG. A novel and easy route to 1,3,4-thiadiazine derivatives via the three- component reaction of phenylhydrazine, α-bromo aryl ketones and aryl isothiocyanates Tetrahedron Lett. 2013;54(46):6215-6217. doi:10.1016/j.tetlet.2013.09.008
Gazieva GA, Kravchenko AN. Thiosemicarbazides in the synthesis of five- and six-membered heterocyclic compounds. Russ Chem Rev. 2012;81(6):494–523 doi:10.1070/RC2012v081n06ABEH004235
Kazakov VYa, Postovskii IYa. Synthesis and some reactions of 4-substituted thiosemicarbazides. Dokl Akad Nauk SSSR. [Internet] 1960 [cited 2024];134 (4):824–827. Russian. Available from: https://www.mathnet.ru/eng/dan24105 Accessed on 15 July 2024
Sarapultsev AP, Chupakhin ON, Sarapultsev PA, Sidorova LP, Tseitler TA. Pharmacologic Evaluation of Antidepressant Activity and Synthesis of 2-Morpholino-5-phenyl-6H-1,3,4-thiadiazine Hydrobromide. Pharmaceuticals. 2016;9(2):27. doi:10.3390/ph9020027
Knak S, Pfeiffer W-D. Synthesis of Imidazo[2,1-b][2H-1,3,4]thiadiazines and 1,2,4-Triazolo[3,4-b][2H-1,3,4]thiadiazines. J Heterocycl Chem. 2015;52(2):463-467. doi:10.1002/chin.201532198
Furukawa M, Kabashima S, Okawara T, Yamasaki T. Synthesis of novel 1,3-thiazolidines and 1,3,4-thiadiazolines from thiocarbohydrazides. Heterocycles. 1990;31(6):1129-1139. doi:10.3987/COM-90-5380
Metwally MA, Bondock S, El-Azap H, Kandeel EM. Thiosemicarbazides:Synthesis and reactions. J Sulfur Chem. 2011;32(5):489-519. doi:10.1080/17415993.2011.601869
Novikova AP, Perova NM, Egorova LG, Bragina EI. Synthesis and properties of 1,3,4-thiadiazine derivatives. 1. Investigation of the condensation of substituted phenacyl bromides and bromoacetylpyridines with thiosemicarbazide. Chem Heterocycl Compd. 1991;27:666–668. doi:10.1007/BF00472523
Lehmann F, Hartmann R, Malmström J, Sibley G. A Versatile One-Pot Procedure for the Synthesis of 5-Aryl-6H-1,3,4-thiadiazine-2-amines from Aromatic Ketones. Synlett. 2016;27(06):864–867. doi:10.1055/s-0035-1561332
Pfeiffer W-D, Gille H, Bulka E, Saghyan A, Langer P. Cyclocondensations of Substituted Thiosemicarbazides with 2-Bromo-1,2-diphenylethan-1-one. Z Naturforschung B. 2013;68(7):823-830. doi:10.5560/znb.2013-3036
Rádl S. Preparation of Some Pyrazole Derivatives by Extrusion of Elemental Sulfur from 1,3,4-Thiadiazines. Collect. Czech. Chem. Commun. 1992;57(3):656-659. doi:10.1135/cccc19920656
Hassan A, Mourad AE, El-Shaieb K, Abou-Zied AH. Synthesis of 1,3,4-Thiadiazole, 1,3,4-Thiadiazine, 1,3,6-Thiadiazepane and Quinoxaline Derivatives from Symmetrical Dithiobiureas and Thioureidoethylthiourea Derivatives. Molecules. 2005;10(7):822-832. doi:10.3390/10070822
Čačič M, Pavić V. Design and Synthesis of Some New 1,3,4-Thiadiazines with Coumarin Moieties and Their Antioxidative and Antifungal Activity. Molecules. 2014;19(1):1163-1177. doi:10.3390/molecules19011163
Schmidt RR, Huth H. Ringverengung bei 6H-1.3.4-Thiadiazinen. Tetrahedron Lett. 1975;16(1):33-36. doi:10.1016/S0040-4039(00)71770-9
DOI: https://doi.org/10.15826/chimtech.2024.11.4.13
Copyright (c) 2024 Tatiana A. Tseitler, Larisa P. Sidorova, Alla V. Ivanova, Oleg N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







