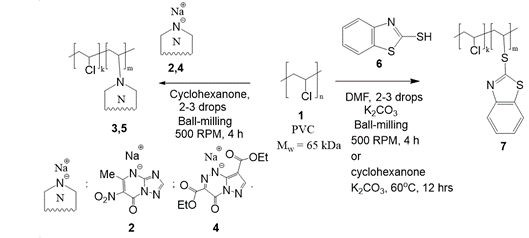
(Mechano)chemical modification of polyvinyl chloride with azole-based drugs
Abstract
Keywords
Full Text:
PDFReferences
Keane MA, Catalytic conversion of waste plastics: focus on waste PVC. J Chem Technol Biotechnol. 2007;82(9):787–795. doi:10.1002/jctb.1757
Kumaga S, Yoshioka T. Feedstock Recycling via Waste Plastic Pyrolysis. J Jpn Pet Inst. 2016;59(6):243–253. doi:10.1627/jpi.59.243
Xu j, Tazawa N, Kumagai S, Kameda T, Saito Y, Yoshioka T. Simultaneous recovery of high-purity copper and polyvinyl chloride from thin electric cables by plasticizer extraction and ball milling. RSC Adv. 2018;8(13):6893–6903. doi:10.1039/C8RA00301G
Born JG, Mulder P, Louw R. Fly ash mediated reactions of phenol and monochlorophenols: oxychlorination, deep oxidation, and condensation. Environ Sci Technol. 1993;27(9):1849–1863. doi:10.1021/es00046a013
Fukushima M, Wu B, Ibe H, Wakai K, Sugiyama E, Abe H, Kitagawa K, Tsuruga S, Shimura K, Ono E. Study on dechlorination technology for municipal waste plastics containing polyvinyl chloride and polyethylene terephthalate. J Mater Cycles Waste Manage. 2010;12(2):108–122. doi:10.1007/s10163-010-0279-8
Lieberzeit P, Bekchanov D, Mukhamediev M. Polyvinyl chloride modifications, properties, and applications: Review. Polym Adv Technol. 2022;33(6):1809–1820. doi:10.1002/pat.5656
Moulay S. Chemical modification of poly(vinyl chloride) – Still on the run. Prog Polym Sci. 2010;35(3):303–331. doi:10.1016/j.progpolymsci.2009.12.001
Hasan AA, Al-Mashhadani MH, Al-Dahhan WH, Yusop RM, Yousif EA. Recent Approaches to Modify Poly(vinyl chloride) Chemical Structure. ANJS. 2022;25(3):8–15. doi:10.22401/ANJS.25.3.02
Shaglaeva NS, Sultangareev RT, Zabanova EA, Lebedeva OV, Trofimova KS. Nucleophilic Substitution of Chlorine Atoms in Polyvinyl Chloride. Russ J Appl Chem. 2008;81(1):131–134. doi:10.1134/S1070427208010291
Kruglova VA, Dobrynina LM, Vereshchagin LI. Synthesis of N-vinylazole polymers via chemical modification of poly(vinylhalides). Polym Sci Ser A. 2007;49(4):407–411. doi:10.1134/S0965545X07040074
Martinez G, Garcia С, Guarrotxena N, Millan J. Local chain configuration dependence of the mechanisms of analogous reactions of PVC-5. Novel results on substitution reaction with sodium 2-mercaptobenzothiazolate. Polymer. 1999;40(6):1507–1514. doi:10.1016/S0032-3861(98)00350-4
Herrero M, Quéméner E, Ulvé S, Reinecke H, Mijangos C, Grohens Y. Bacterial adhesion to poly(vinyl chloride) films: effect of chemical modification and water induced surface reconstruction. J Adhesion Sci Technol. 2006;20(2–3):183–195. doi:10.1163/156856106775897801
Abdelaal MY, Sobahi TR. Chemical modification of PVC intopolymer-supported oxazolinones and triazoles. J Appl Polym Sci. 2007;104(4):2304–2309. doi:10.1002/app.25694
Meier MR, Schubert US. Terpyridine-modified poly(vinyl chloride):possibilities for supramolecular grafting and crosslinking. J Polym Sci A Polym Chem. 2003;41(19):2964–2973. doi:10.1002/pola.10881
Lia J, Yua F, Chena Y, Oupickya D. Polymeric drugs: Advances in the development of pharmacologically active polymers. J Control Release. 2015;219:369–382. doi:10.1016/j.jcorel.2015.09.043
Azam MA, Suresh B. Biological Activities of 2-Mercaptobenzothiazole Derivatives: A Review. Sci Pharm. 2012;80(4):789–823. doi:10.3797/scipharm.1204-27
Rusinov VL, Sapozhnikova IM, Bliznik AM, Chupakhin ON, Charushin VN, Spasov AA, Vassiliev PM, Kuznetsova VA, Rashchenko AI, Babkov DA. Synthesis and Evaluation of Novel [1,2,4]Triazolo[5,1-c][1,2,4]-triazines and Pyrazolo[5,1-c][1,2,4]triazines as Potential Antidiabetic Agents. Arch Pharm Chem Life Sci. 2017;350(5):e1600361. doi:10.1002/ardp.201600361
Baklykov AV, Tumashov AA, Kotovskaya SK, Ulomsky EN, Rusinov GL, Rusinov VL, Artem’ev GA, Kopchuk DS, Charushin VN. Method of determination of 5-methyl-6-nitro-7-oxo-1,2,4-triazolo[1,5-a]pirimidinide l-argininy - the active component of drug «triazid» by HPLC method. Drug Dev Regist. 2018;2(23):78–83.
Lee JW, Park J, Lee J, Park S, Kim JG, Kim BS. Solvent‐Free Mechanochemical Post‐Polymerization Modification of Ionic Polymers. ChemSusChem. 2021;14(18):3801–3805. doi:10.1002/cssc.202101131
Ohn N, Kim JG. Mechanochemical Post-Polymerization Modification: Solvent-Free Solid-State Synthesis of Functional Polymers. ACS Macro Lett. 2018;7(5):561–565. doi:10.1021/acsmacrolett.8b00171
Choudhury N, Kim A, Kim M, Kim BS. Mechanochemical Degradation of Poly (vinyl chloride) into Nontoxic Water‐Soluble Products via Sequential Dechlorination, Heterolytic Oxirane Ring‐Opening, and Hydrolysis. Adv Mater. 2023;35(33):2304113. doi:10.1002/adma.202304113
Kameda T, Ono M, Grause G, Mizoguchi T, Yoshioka T. Ball mill-assisted dechlorination of flexible and rigid poly (vinyl chloride) in NaOH/EG solution. Ind Eng Chem Res. 2008;47(22):8619–8624. doi:10.1021/ie8006819
DOI: https://doi.org/10.15826/chimtech.2024.11.2.11
Copyright (c) 2024 Eman S.A. Al-Sammarraie, Wahab A.K. Al-Ithawi, Artem V. Baklykov, Vadim A. Platonov, Aqeel M.K. Altobee, Nikita S. Glebov, Albert F. Khasanov, Igor S. Kovalev, Igor L. Nikonov, Dmitry S. Kopchuk, Irina Sapozhnikova, Tamara M. Sabirova, Yanxian Jin et al.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







