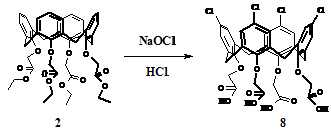
The synthesis and alkylation of p-tetrakischloro-calix[4]arene
Abstract
Keywords
Full Text:
PDF (RUSSIAN) (Русский)References
Kumar S, Chawla HM, Varadarajan R. A one-step, one-pot synthesis of p-acyl calix[n]arenes. Tetrahedron Lett. 2002;43(14):2495-98. doi:10.1016/S0040-4039(02)00325-8
Hapiot F, Lyskawa J, Bricout H, Tilloy S, Monflier E. Cyclodextrins or Calixarenes: What is the Best Mass Transfer Promoter for Suzuki Cross-Coupling Reactions in Water?. Adv Synth Catal. 2004;346(1):83-9. doi:10.1002/adsc.200303149
Gunji A, Takahashi K. Selective and Efficient Iodination of the p-Positions of Calix[4]arene Derivatives. Synth Commun. 1998;28(21):3933-41. doi:10.1080/00397919808004951
Carroll LT, Hill PA, Ngo CQ, Klatt KP, Fantini JL. Synthesis and reactions of a 2-chlorocalix[4]arene and a 2,2′-coupled dicalixarene. Tetrahedron. 2013;69:5002-7. doi:10.1016/j.tet.2013.04.010
Peles-Lemli B, Peles-Lemli J, Bitter I, Kollár L, Nagy G, Kunsági-Máté SJ. Competitive thermodynamic and kinetic processes during dissociation of some host-guest complexes of calix[4]arene derivatives. Incl Phen Macrocycl Chem. 2007;59:251-6. doi:10.1007/s10847-007-9322-3
DOI: https://doi.org/10.15826/chimtech.2014.1.1.729
Copyright (c) 2014 E. A. Ivanova, T. V. Glukhareva, Yu. Yu. Morzherin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






