Novel direct synthetic route of 2D Prussian Blue analogue, nanocrystalline CuHCF, as highly effective cathode materials for Zn-ion supercapacitors
Abstract
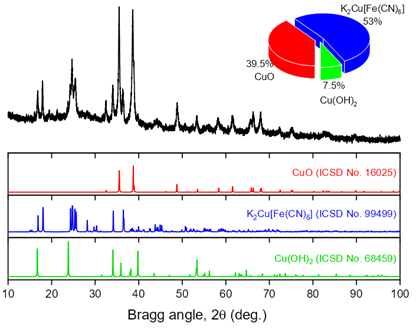
Prussian blue analogues (PBAs) with 2D morphology of nanocrystals have attracted much attention for aqueous electrolyte-based energy storage devices. In this study, we synthesized a 2D Prussian blue analogue, nanocrystals of copper hexacyanoferrate (CuHCF), via a facile stepwise route involving a modified copper substrate of Cu(OH)2 nanorods that was used for the formation of two-dimensional CuHCF crystals. These materials were characterized by powder X-ray diffraction, energy dispersive X-ray microanalysis, X-ray photoelectron spectroscopy and scanning electron microscopy. The cathode based on 2D CuHCF exhibits high specific capacity (240 F/g (63.9 mAh/g) at 0.1 A/g) with excellent cycling stability (98.5% retention after 1000 charge-discharge cycles) in 3 M ZnSO4 electrolyte. The flat two-dimensional morphology of CuHCF provides sufficient ion diffusion channels and the numerous electroactive interfaces for intercalation charge storage.
Keywords
Full Text:
PDFReferences
Zhu K, Wu T, Sun S, Wen Y, Huang K. Electrode materials for practical rechargeable aqueous Zn-ion batteries: challenges and opportunities. ChemElectroChem 2020;7:2714–2734. doi:10.1002/celc.202000472
He P, Chen Q, Yan M, Xu X, Zhou L. Building better zinc ion batteries: a materials perspective. EnergyChem 2019;1(3):100022. doi:10.1016/j.enchem.2019.100022
Li C, Zhang X, He W, Xu G, Sun R. Cathode materials for rechargeable zinc-ion batteries: From synthesis to mechanism and applications. J Power Sources. 2020;449:227596. doi:10.1016/j.jpowsour.2019.227596
An G, Hong J, Pak S, Cho Y, Lee S. 2D metal Zn nanostructure electrodes for high performance Zn ion supercapacitors. Adv Energy Mater. 2020;10(3):1902981. doi:10.1002/aenm.201902981
Zhang P, Li Y, Wang G, Wang F, Yang S. Zn ion hybrid micro supercapacitors with ultrahigh areal energy density and long term durability. Adv Mater. 2019;31(3):1806005. doi:10.1002/adma.201806005
Wu S, Chen Y, Jiao T, Zhou J, Cheng J. An aqueous Zn ion hybrid supercapacitor with high energy density and ultrastability up to 80 000 cycles. Adv Energy Mater. 2019;9(47):1902915. doi:10.1002/aenm.201902915
Chao D, Fan HJ. Intercalation pseudocapacitive behavior powers aqueous batteries. Chem. 2019;5(6):1359–1361. doi:10.1016/j.chempr.2019.05.020
Tang H, Yao J, Zhu Y. Recent developments and future prospects for zinc ion hybrid capacitors: a review. Adv Energy Mater. 2021;11(14):2003994. doi:10.1002/aenm.202003994
Jin J, Geng X, Chen Q, Ren TL. A better Zn Ion storage device: Recent progress for Zn Ion hybrid supercapacitors. Nano Micro Lett. 2022;14:64 doi:10.1007/s40820 022 00793w
Lobinsky A, Kaneva M, Tenevich M, Popkov V. Direct synthesis of Mn3[Fe(CN)6]2·nH2O nanosheets as novel 2D analog of Prussian Blue and material for high-performance metal-ion batteries. Micromachines. 2023;14(5):1083. doi:10.3390/mi14051083
Lin Y, Zhang L, Xiong Y, Wei T, Fan Z. Toward the design of high-performance supercapacitors by Prussian Blue, its analogues and their derivatives. Energy Environ Mater. 2020;3:323–345. doi:10.1002/eem2.12096
Hurlbutt K, Wheeler S, Capone I, Pasta M. Prussian Blue analogs as battery materials. Joule. 2018;2:1950–1960 doi:10.1016/j.joule.2018.07.017
Cao L-M, Lu D, Zhong D-C, Lu T-B. Prussian blue analogues and their derived nanomaterials for electrocatalytic water splitting. Coordination Chem Rev. 2020;407:213156. doi:10.1016/j.ccr.2019.213156
Ying S, Chen C, Wang J, Lu C, Liu T, Kong Y, Yi F-Y. Synthesis and applications of Prussian Blue and its analogues as electrochemical sensors. ChemPlusChem. 2021;86:1608–1622. doi:10.1002/cplu.202100423
Holland A, Kimpton H, Cruden A, Wills R. CuHCF as an electrode material in an aqueous dual-ion Al3+/K+ ion battery. Energy Procedia. 2018;151:69–73 doi:10.1016/j.egypro.2018.09.029
Liu S, Pan G-L, Lia G-R, Gao XP. Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J Mater Chem A. 2015;3:959–962. doi:10.1039/C4TA04644G
Song Z, Liu W, Wei X, Zhou Q, Liu H, Zhang Z, Liu G, Zhao Z. Charge storage mechanism of copper hexacyanoferrate nanocubes for supercapacitors. Chin Chem Lett. 2020;31(5):1213–1216. doi:10.1016/j.cclet.2019.07.022
Yilmaz G, Tan CF, Hong M, Ho GW. Functional defective metal-organic coordinated network of mesostructured nanoframes for enhanced electrocatalysis. Adv Funct Mater. 2018;28:1704177. doi:10.1002/adfm.201704177
Shen L, Zhang Q, Luo J, Fu HC, Chen XH, Wu LL, Luo HQ, Li NB. Fabrication of 2D/3D hierarchical PBA and derivative electrocatalysts for overall water splitting. Appl Surf Sci. 2021;551:149360. doi:10.1016/j.apsusc.2021.149360
Yin J, Zhou J, Wang Y, Ma Y, Zhou X, Wang G, Yang Y, Lu P, Yu J, Chen Y, Yuan Y, Ye C, Xi S, Fan Z. Controlled synthesis of 2D Prussian Blue analog nanosheets with low coordinated water content for high-performance lithium storage. Small Methods. 2022;2201107. doi:10.1002/smtd.202201107
Liu T, Wang J, Jiang Q, Chai N, Ying S, Konga Y, Yi F-Y. One-step synthesis of 2D@3D hollow Prussian blue analogue as a high-performance bifunctional electrochemical sensor. Dalton Trans. 2023;52:9048–9057. doi:10.1039/D3DT00957B
Wang Y, Ma J, Wang J, Chen S, Wang H, Zhang J. Interfacial scaffolding preparation of hierarchical pba-based derivative electrocatalysts for efficient water splitting. Adv Energy Mater. 2019;9:1802939. doi:10.1002/aenm.201802939
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RSC. Resolving surface chemical states in XPS analysis of first-row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci. 2011;257:2717–2730. doi:10.1016/j.apsusc.2010.10.051
Song Z, Liub W, Weia X, Zhoua Q, Liub H, Zhang Z, Liu G, Zhao Z. Charge storage mechanism of copper hexacyanoferrate nanocubes for supercapacitors. Chin Chem Lett. 2020;31(5):1213–1216. doi:10.1016/j.cclet.2019.07.022
DOI: https://doi.org/10.15826/chimtech.2023.10.4.19
Copyright (c) 2023 Maria V. Kaneva, Anastacia K. Bachina, Artem A. Lobinsky

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







