Conjugates of 8-[2,2’-bipyridinyl]coumarins as potential chemosensors for Al3+, Cu2+, Cd2+, Zn2+ ions: synthesis and photophysical properties
Abstract
Keywords
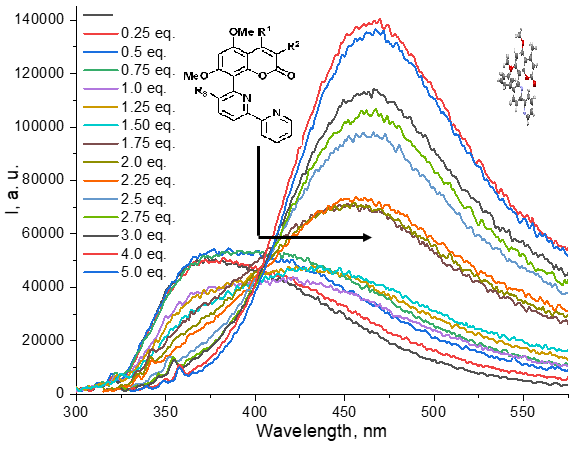
Full Text:
PDFReferences
Cao D, Liu Z, Verwilst P, Koo S, Jangjili P, Kim JS, Lin W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chemical Reviews. 2019;119(18):10403–10519. doi:10.1021/acs.chemrev.9b00145
Górski K, Deperasińska I, Baryshnikov GV, Ozaki S, Kamada K, Ågren H, Gryko DT. Quadrupolar dyes based on highly polarized coumarins. Org Lett. 2021;23(17):6770–6774. doi:10.1021/acs.orglett.1c02349
Shaydyuk YO, Bashmakova NV, Klishevich GV, Dmytruk AM, Kachkovsky OD, Kuziv IB, Dubey IY, Belfield KD, Bondar MV. Nature of linear spectral properties and fast relaxations in the excited states and two-photon absorption efficiency of 3-thiazolyl and 3-phenyltiazolyl coumarin derivatives. ACS Omega. 2023;8(12):11564–11573. doi:10.1021/acsomega.3c00654
Verma P, Pal, H. Aggregation Studies of Dipolar Coumarin-153 Dye in Polar Solvents: A Photophysical Study. J Phys Chem A. 2014;118(34):6950–6964. doi:10.1021/jp506138w
Jana K, Sarkar D, Jaiswal P, Moorthy JN. Synthesis and Excited-State Properties of Donor–Acceptor Azahelical Coumarins. J Org Chem. 2023;88(11):6611-6622. doi:10.1021/acs.joc.2c02810
Xu Z, Chen X, Kim HN, Yoon J. Sensors for the optical detection of cyanide ion. Chem Soc Rev. 2010;39:127–137. doi:10.1039/B907368J
Zhou Y, Zhang JF, Yoon J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem Rev. 2014;114:5511–5571. doi:10.1021/cr400352m
Kim SK, Lee DH, Hong JI, Yoon J. Chemosensors for pyrophosphate. Acc Chem Res. 2009;42:23–31. doi:10.1021/ar800003f
Wu J, Kwon B, Liu W, Anslyn EV, Wang P, Kim JS. Chromogenic/fluorogenic ensemble chemosensing systems. Chem Rev. 2015;115:7893–7843. doi:10.1021/cr500553d
Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Artificial receptors for the recognition of phosphorylated molecules. Chem Rev. 2011;111:6603–6782. doi:10.1021/cr100242s
Shiraishi Y, Nakamura M, Hayashi N, Hirai T. Coumarin-spiropyran dyad with a hydrogenated pyran moiety for rapid, selective, and sensitive fluorometric detection of cyanide anion. Anal Chem. 2016;88:6805–6811. doi:10.1021/acs.analchem.6b01279
Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev. 2008;108:1517–1549. doi:10.1021/cr078203u
Verwilst P, Sunwoo K, Kim JS. The role of copper ions in pathophysiology and fluorescent sensors for the detection thereof. Chem Commun. 2015;51:5556–5571. doi:10.1039/C4CC10366A
Chen X, Pradhan T, Wang F, Kim JS, Yoon J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev. 2012;112:1910–1956. doi:10.1021/cr200201z
Zhang JF, Zhou Y, Yoon J, Kim JS. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem Soc Rev. 2011;40:3416–3429. doi:10.1039/c1cs15028f
Qian X, Xu Z. Fluorescence imaging of metal ions implicated in diseases. Chem Soc Rev. 2015;44:4487–4493. doi:10.1039/C4CS00292J
Sun W, Guo S, Hu C, Fan J, Peng X. Recent development of chemosensors based on cyanine platforms. Chem Rev. 2016;116:7768–7817. doi:10.1021/acs.chemrev.6b00001
Dong B, Song X, Kong X, Wang C, Tang Y, Liu Y, Lin W. Simultaneous near-infrared and two-photon in vivo imaging of H2O2 using a ratiometric fluorescent probe based on the unique oxidative rearrangement of oxonium. Adv Mater. 2016;28:8755–8759. doi:10.1002/adma.201602939
Men Y, Li Z, Zhang J, Tong Z, Xi Z, Qiu X, Yi L. Rational design and synthesis of fast-response NBD-based fluorescent probes for biothiols. Tetrahedron Lett. 2015;56:5781–5786. doi:10.1016/j.tetlet.2015.08.073
He L, Yang X, Xu K, Yang Y, Lin W. A multifunctional logic gate by means of a triple-chromophore fluorescent biothiol probe with diverse fluorescence signal patterns. Chem Commun. 2017;53:13168–13171. doi:10.1039/C7CC07296A
Manjare ST, Kim Y, Churchill DG. Selenium- and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc Chem Res. 2014;47:2985–2998. doi:10.1021/ar500187v
Dong B, Zheng K, Tang Y, Lin W. Development of green to near-infrared turn-on fluorescent probes for the multicolour imaging of nitroxyl in living systems. J Mater Chem B. 2016;4:1263–1269. doi:10.1039/C5TB02073E
Zhang Q, Zhu Z, Zheng Y, Cheng J, Zhang N, Long Y-T, Zheng J, Qian X, Yang Y. A three-channel fluorescent probe that distinguishes peroxynitrite from hypochlorite. J Am Chem Soc. 2012;134:18479–18482. doi:10.1021/ja305046u
Signore G, Nifosì R, Albertazzi L, Storti B, Bizzarri R. Polarity-sensitive coumarins tailored to live cell imaging. J Am Chem Soc. 2010;132:1276–1288. doi:10.1021/ja9050444
Hirosawa S, Arai S, Takeoka S. A TEMPO-conjugated fluorescent probe for monitoring mitochondrial redox reactions. Chem Commun. 2012;48:4845–4847. doi:10.1039/c2cc30603d
Xiao-ya S, Teng L, Jie S, Xiao-jing W. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020;10:10826–10847. doi:10.1039/C9RA10290F
Li T, Fang R, Wang B, Shao Y, Liu J, Zhang S, Yang Z. A simple coumarin as a turn-on fluorescence sensor for Al(III) ions. Dalton Trans. 2014;43:2741–2743. doi:10.1039/C3DT52414K
Guha S, Lohar S, Sahana A, Banerjee A, Safin DA, Babashkina MG, Mitoraj MP, Bolte M, Garcia Y, Mukhopadhyay SK, Das D. A coumarin-based “turn-on” fluorescent sensor for the determination of Al3+: single crystal X-ray structure and cell staining properties. Dalton Trans. 2013;42:10198–10207. doi:10.1039/c3dt51045j
García-Beltrán O, Cassels BK, Mena N, Nuñez MT, Yañez O, Caballero J. A coumarinylaldoxime as a specific sensor for Cu2+ and its biological application. Tetrahedron Lett. 2014;55:873–876. doi:10.1016/j.tetlet.2013.12.033
Kumari C, Sain D, Kumar A, Debnath S, Saha P, Dey S. Intracellular detection of hazardous Cd2+ through a fluorescence imaging technique by using a nontoxic coumarin based sensor. Dalton Trans. 2017;46:2524–2531. doi:10.1039/C6DT04833A
Maity D, Govindaraju T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem Commun. 2012;48:1039–1041. doi:10.1039/C1CC16064
Fatykhov RF, Khalymbadzha IA, Sharapov AD, Potapova AP, Starnovskaya ES, Kopchuk DS, Chupakhin, ON. Expedient synthesis of 1,2,4-triazinyl substituted benzo[c]coumarins via double oxidation strategy. Chim Tech Acta. 2023;10(2):202310205. doi:10.15826/chimtech.2023.10.2.05
Fatykhov RF, Savchuk MI, Starnovskaya ES, Bobkina MV, Kopchuk DS, Nosova EV, Zyryanov GV, Khalymbadzha IA, Chupakhin ON, Charushin VN, Kartsev, VG. Nucleophilic substitution of hydrogen–the Boger reaction sequence as an approach towards 8-(pyridin-2-yl)coumarins. Mendeleev Commun. 2019;29(3):299–300. doi:10.1016/j.mencom.2019.05.019
Fatykhov RF, Sharapov AD, Starnovskaya ES, Shtaitz, YK, Savchuk MI, Kopchuk DS, Nikonov IL, Zyryanov GV, Khalymbadzha IA, Chupakhin ON. Coumarin-pyridine push-pull fluorophores: Synthesis and photophysical studies. Spectrochim Acta A Mol Biomol Spectros. 2022;267:120499. doi:10.1016/j.saa.2021.120499
Sharapov AD, Fatykhov RF, Khalymbadzha IA, Sharutin VV, Santra S, Zyryanov G, Chupakhin ON, Ranu BC. Mechanochemical synthesis of coumarins via Pechmann condensation under solvent-free conditions: an easy access to coumarins and annulated pyrano[2,3-f] and [3,2-f]indoles. Green Chem. 2022;4(6):2429–2437. doi:10.1039/d1gc04564d
Santra S, Sharapov AD, Fatykhov RF, Potapova AP, Khalymbadzha IA, Valieva MI, Kopchuk DS, Zyryanov GV, Bunev AS, Melekhin VV, Gaviko VS, Zonov AA. Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and anticancer activity. Pharmaceut. 2023;16(3):403. doi:10.3390/ph16030403
Shabunina OV, Kopchuk DS, Ustinova MM, Kozhevnikov DN, Kozhevnikov VN, König B. Facile Synthesis Of 6-Aryl-3-Pyridyl-1,2,4-Triazines As A Key Step Toward Highly Fluorescent 5-Substituted Bipyridines And Their Zn(II) And Ru(II) Complexes. Tetrahedron. 2008;64(37):8963–8973. doi:10.1016/j.tet.2008.06.040
Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer. Boston, 2006. 205–235 p. doi:10.1007/978-0-387-46312-4
Von Lippert EZ. Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Ber Bunsenges. Phys Chem. 1957;61(8):962–975. doi:10.1002/bbpc.19570610819
Mataga N, Kaifu Y, Koizumi M. Solvent effects uponfluorescence spectra and thedipolemoments of excited molecules. Bull Chem Soc Jpn. 1956;29:465–470. doi:10.1246/BCSJ.29.465
Kawski A. On the estimation of excited-state dipole moments from solvatochromicshifts of absorption andfluorescence spectra, Z. Naturforsch A. 2002;57(5):255–262. doi:10.1515/zna-2002-0509
Rabek JF. Progress in photochemistry and photophysics, vol. V. CRC Press. Boca Raton, 2016. 1–208 p.
Kopchuk DS, Chepchugov NV, Starnovskaya ES, Khasanov AF, Krinochkin AP, Santra S, Zyryanov GV, Das P, Majee A, Rusinov VL, Charushin VN. Synthesis and optical properties of new 2-(5- arylpyridine-2-yl)-6-(het)arylquinoline-based ‘‘push-pull” fluorophores. Dyes Pigm. 2019;167:151–156. doi:10.1016/j.dyepig.2019.04.029
Kosower EM. An Introduction to Physical Organic Chemistry. Wiley. New York, 1968. 293 p.
Kosower EM, The Effect of Solvent on Spectra. I. A New Empirical Measure of Solvent Polarity: Z-Values. J Am Chem Soc. 1958;80(13):3253–3260. doi:10.1021/ja01546a020
Dimroth K, Reichardt C, Siepmann T, Bohlmann F. Über Pyridinium-Nphenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, Liebigs. Ann Chem. 1963;661(1):1–37. doi:10.1002/jlac.19636610102
Reichardt C. Über Pyridinium-N-phenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, VI. Erweiterung der Lösungsmittelpolaritätsskala durch Bestimmung neuer molarer Übergangsenergien (ET-Werte), Liebigs. Ann Chem. 1971;752(1):64–67. doi:10.1002/jlac.19717520109
Sheldrick GM. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi:10.1107/S0108767307043930
CrysAlisPro, version 1.171.39.38a, Data Collection, Reduction and Correction Program, Rigaku Oxford Diffraction, 2017.
Sharapov, AD, Fatykhov, RF, Khalymbadzha, IA, Valieva, MI, Nikonov, IL, Taniya, OS, Kopchuk, DS, Zyryanov, GV, Potapova, AP, Novikov, AS, Sharutin, VV, Chupakhin, ON. Fluorescent Pyranoindole Congeners: Synthesis and Photophysical Properties of Pyrano[3,2-f], [2,3-g], [2,3-f], and [2,3-e]Indoles. Molecules. 2022;27(24):8867. doi:10.3390/molecules27248867
DOI: https://doi.org/10.15826/chimtech.2023.10.4.17
Copyright (c) 2023 Ainur D. Sharapov, Ramil F. Fatykhov, Igor A. Khalymbadzha, Dmitry S. Kopchuk, Igor L. Nikonov, Anastasiya P. Potapova, Yaroslav K. Shtaitz, Pavel A. Slepukhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







