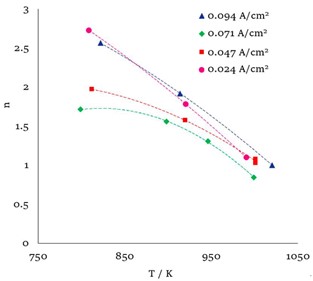
Anodic dissolution of gallium in alkali metal chloride melts
Abstract
Keywords
Full Text:
PDFReferences
Wilson PD. The nuclear fuel cycle: from ore to wastes. Oxford University Press: Oxford; 1996. 323 p. doi:10.1093/oso/9780198565406.001.0001
Nawada HP, Fukuda K. Role of pyrochemical processes in advanced fuel cycles. J Phys Chem Solids. 2005;66:647. doi:10.1016/j.jpcs.2004.07.022
Mendes E, Conocar O, Laplace A, Douyere N, Miguirditchian M. Study of innovative chemical processes for sodium fast reactor fuel assemblies cleaning. Procedia Chem. 2012;7:791. doi:10.1016/j.proche.2012.10.120
Mirza M, Abdulaziz R, Maskell WC, Wilcock S, Jones AH, Woodall S, Jackson A, Shearing PR, Brett DJL. Electrochemical processing in molten salts – a nuclear perspective. Energy Environ Sci. 2023;16:952. doi:10.1039/d2ee02010f
Ignatiev V, Feynberg O, Gnidoi I, Konakov S, Kormilitsyn M, Merzliakov A, Surenkov A, Uglov V, Zagnitko A. MARS: Story on molten salt actinide recycler and transmuter development by Rosatom in co-operation with Euratom in Actinide and Fission Product Partitioning and Transmutation. Thirteenth Information Exchange Meeting, Nuclear Science NEA/NSC/R. 2015;2:92–103.
Allibert M, Delpech S, Gerardin D, Heuer D, Laureau A, Merlea E. Homogeneous molten salt reactors (MSRs): The molten salt fast reactor (MSFR) concept. Handbook of Generation IV Nuclear Reactors: Pioro IL. Woodhead Publishing: Sawston; 2016. 231–257 pp. doi:10.1016/B978-0-12-820588-4.00005-0
Serp J, Allibert M, Benes O, Delpech S, Feynberg O, Ghetta V, Heuer D, Holcomb D, Ignatiev V, Kloosterman JL, Luzzi L, Merle-Lucotte E, Uhlír J, Yoshioka R, Zhimin D. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog Nucl Energy. 2014;77:308. doi:10.1016/j.pnucene.2014.02.014
Bychkov AV, Skiba OV. Review of non-aqueous nuclear fuel reprocessing and separation methods. Chem Sep Technol Related Meth Nuclear Waste Manag. 1999:71–98. doi:10.1007/978-94-011-4546-6_5
Inoue T, Sakamura Y. Pyrochemistry in nuclear industry. Molten Salts Fundamen Appl. 2002:249–261. doi:10.1007/978-94-010-0458-9_9
Jiang D, Zhang D, Li X, Wang S, Wang C, Qin H, Guo Y, Tian W, Su GH, Qiu S. Fluoride-salt-cooled high-temperature reactors: Review of historical milestones, research status, challenges, and outlook. Renewable Sustain Energy Rev. 2022;161. doi:10.1016/j.rser.2022.112345
Morgan LG, Burger LL, Scheele RD. Molten salt oxidation-reduction processes for fuel processing. Actinide Separat ACS Symposium Ser. 1979;117:233–252. doi:10.1021/bk-1980-0117.ch017
Brambilla G, Facchini AG. U-Pu recovery by molten alkaline sulphates. Radiochim Acta. 1984;36:37. doi:10.1524/ract.1984.36.12.37
Griffiths TR, Volkovich VA, Yakimov SM, May I, Sharrad CA, Charnock JM. Reprocessing spent nuclear fuel using molten carbonates and subsequent precipitation of rare earth fission products using phosphate. J Alloys Comp. 2006;418:116–121. doi:10.1016/j.jallcom.2005.10.060
Volkovich, VA, Maltsev DS, Raguzina EV, Dedyukhin AS, Shchetinskiy AV, Yamshchikov LF, Chukin AV. Thermodynamics of rare earth elements and uranium in gallium based quaternary metallic alloys. J Alloys Compd. 2019;787:367–378. doi:10.1016/j.jallcom.2019.02.081
Xu H, Zhang W, Wang C, Yang M, Yan T, Yan Y, Zhang M. Molten salt/liquid metal extraction: electrochemical behaviors and thermodynamics properties of La, Pr, U and separation factors of La/U and Pr/U couples in liquid gallium cathode. Appl Radiat Isot. 2022;182. doi:10.1016/j.apradiso.2022.110149
Liu K, Chai ZF, Shi WQ. Liquid electrodes for An/Ln separation in pyroprocessing. J Electrochem Soc. 2021;168. doi:10.1149/1945-7111/abec99
Volkovich VA, Maltsev DS, Melchakov SY, Yamshchikov LF, Novoselova AV, Smolensky VV. Separation of lanthanides and actinides in a chloride melt – liquid metal system: the effect of phase composition. ECS Trans. 2016;75:397–408. doi:10.1149/07515.0397ecst
Dedyukhin AS, Shchetinskiy AV, Kharina EA, Shchepin IE, Volkovich VA, Yamshchikov LF, Osipenko AG. Electrochemical and thermodynamic properties of lanthanum in a chloride melt – liquid metal system. ECS Trans. 2016;75:265–274. doi:10.1149/07515.0265ecst
Smolenski V, Novoselova A, Osipenko A, Maershin A. Thermodynamics and separation factor of uranium from lanthanum in liquid eutectic gallium-indium alloy/molten salt system. Electrochim Acta. 2014;145:81–85. doi:10.1016/j.electacta.2014.08.081
Smolenski V, Novoselova A, Volkovich VA. Thermodynamics of La and U and the Separation Factor of U/La in Fused Me(Ga-40 wt.% In)/3LiCl–2KCl System. J Nucl Mater. 2017;495:285–290. doi:10.1016/j.jnucmat.2017.08.017
Novoselova A, Smolenski V, Volkovich VA, Luk’yanova Y. Thermodynamic Properties of Ternary Me-Ga-In (Me = La, U) Alloys in a Fused Ga-In/LiCl-KCl System. J Chem Thermodyn. 2019;130:228–234. doi:10.1016/j.jct.2018.10.014
Novoselova A, Smolenski V, Volkovich VA, Ivanov AB, Osipenko A, Griffiths TR. Thermodynamic properties of La–Ga–Al and U–Ga–Al Alloys and the separation Factor of U/La couple in the molten salt–liquid metal system. J Nucl Mater. 2015;466:373–378. doi:10.1016/j.jnucmat.2015.08.010
Dedyukhin AS, Kharina EA, Raguzina EV, Maltsev DS, Shchetinskiy AV, Volkovich VA, Yamshchikov LF. Solubility of lanthanum and uranium in Ga–In and Ga–Al eutectic based alloys. AIP Conf Proc. 2018;2015:020019. doi:10.1063/1.5055092
Volkovich VA, Maltsev DS, Yamshchikov LF, Osipenko AG. Thermodynamic properties of uranium in liquid gallium, indium and their alloys. J Nucl Mater. 2015;464:263–269. doi:10.1016/j.jnucmat.2015.04.054
Dedyukhin AS, Shchetinskiy AV, Volkovich VA, Yamshchikov LF, Osipenko AG. Lanthanum activity, activity coefficients and solubility in gallium-indium liquid alloys. ECS Trans. 2014;64:227–234. doi:10.1149/06404.0227ecst
Shchetinskiy AV, Dedyukhin AS, Volkovich VA, Yamshchikov LF, Maisheva AI, Osipenko AG, Kormilitsyn MV. Thermodynamic properties of lanthanum in gallium–indium eutectic based alloys. J Nucl Mater. 2013;435:202–206. doi:10.1016/j.jnucmat.2012.12.035
Dedyukhin AS, Shepin IE, Kharina EA, Shchetinskiy AV, Volkovich VA, Yamshchikov LF. Thermodynamic properties of lanthanum in gallium–zinc alloys. AIP Conf Proceed. 2016;1767:020006. doi:10.1063/1.4962590
Novoselova A, Smolenski V. The influence of the temperature and Ga-In alloy composition on the separation of uranium from neodymium in molten Ga-In/3LiCl-2KCl system during the recycling of high-level waste. J Nucl Mater. 2018;509:313–317. doi:10.1016/j.jnucmat.2018.06.040
Smolenski V, Novoselova A, Osipenko A, Kormilitsyn M, Luk’Yanova Y. Thermodynamics of separation of uranium from neodymium between the gallium-indium liquid alloy and the LiCl-KCl molten salt phases. Electrochim Acta. 2014;133:354–358. doi:10.1016/j.electacta.2014.04.042
Smolenski V, Novoselova A, Volkovich V, Luk’yanova Y, Osipenko A, Bychkov A, Griffiths TR. The effect of Al concentration on thermodynamic properties of Nd and U in Ga–Al-based alloys and the separation factor of Nd/U couple in a “molten salt-liquid metal system”. J Radioanal Nucl Chem. 2017;311:687–693. doi:10.1007/s10967-016-5053-5
Schetinskiy AV, Dedyukhin AS, Kharina EA, Volkovich VA, Yamshchikov LF. Activity coefficients of lanthanum in gallium and gallium-aluminum based alloys. J Alloys Compd. 2019;790:809–813. doi:10.1016/j.jallcom.2019.03.199
Boudraa S, Djaballah Y, Mansouri Y, Belgacem Bouzida A. Thermodynamic assessment of the Ga–La and Ga–Pr systems supported by ab-initio calculations. Calphad. 2022;76:102387. doi:10.1016/j.calphad.2021.102387
Smolenski VV, Novoselova AV, Bovet AL, Mushnikov PN. Separation factors of La/U, Pr/U, and Nd/U in the Ga–In/3LiCl–2KCl molten system. Russ Metall. 2020;2020:112–114. doi:10.1134/S0036029520020135
Liu K, Liu YL, Chai ZF, Shi WQ. Electroseparation of uranium from lanthanides (La, Ce, Pr, Nd and Sm) on liquid gallium electrode. Sep Purif Technol. 2021;265:118524. doi:10.1016/j.seppur.2021.118524
Usov PM, Saltykova EA. Termodinamika obrazovaniya hloridov galliya v rasplave. [Thermodynamics of the formation of gallium chlorides in the melt.] Rasplavy. 1987;1(3):110–113.
Tokarev OV, Volkovich VA, Ryzhov AA, Maltsev DS. Electrode potentials of gallium in fused alkali chlorides. ECS Trans. 2022;109(14):197–204. doi:10.1149/10914.0197ecst
Tokarev OV, Maltsev DS, Volkovich VA. Electrochemical properties of gallium in molten alkali metal chlorides. AIP Conf Proc. 2020;2313:020005. doi:10.1063/5.0032401
DOI: https://doi.org/10.15826/chimtech.2023.10.4.16
Copyright (c) 2023 Oleg V. Tokarev, Vladimir A. Volkovich

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







