Novel Nb5+-doped hexagonal perovskite Ba5In2Al2ZrO13 (structure, hydration, electrical conductivity)
Abstract
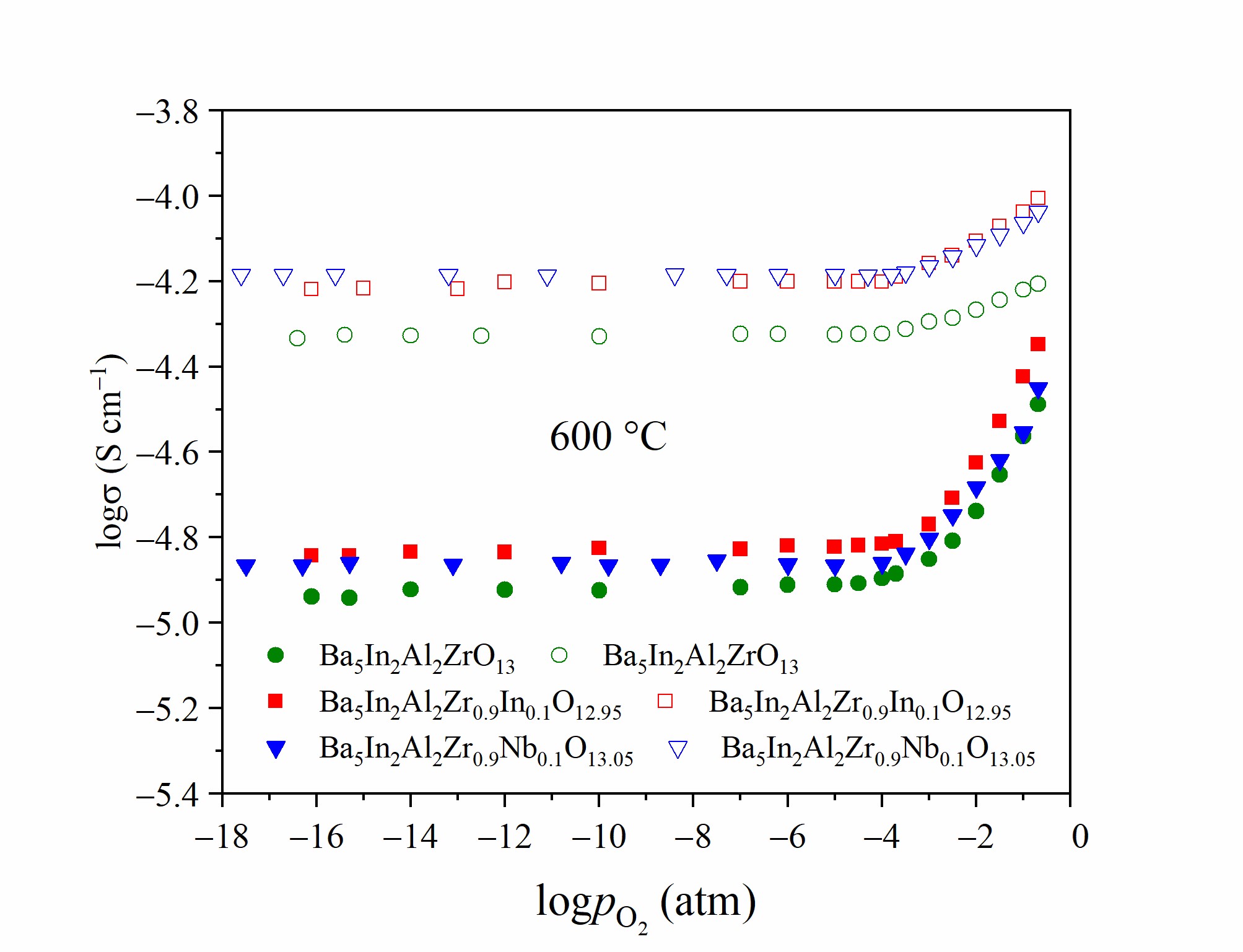
OH−-groups with different thermal stability, which participate in different hydrogen bonds. The new phase Ba5In2Al2Zr0.9Nb0.1O13.05 demonstrates the predominant protonic conductivity at pH2O = 2·10−2 atm and Т<600 °C.
Keywords
Full Text:
PDFReferences
Malavasi L, Fisher CAJ, Islam MS. Oxide-ion and proton conducting electrolyte materials for clean energy applica-tions: structural and mechanistic features. Chem Soc Rev. 2010;39:4370–4387. doi:10.1039/B915141A
Mengfei Z, Georgina J, Peimiao Z, Rong L, Mingtai W, Huanting W, Shanwen T. Recent development of perovskite oxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Ma-ter Today. 2021;49:351–377. doi:10.1016/j.mattod.2021.05.004
Mauro C, Maths K, Lorenzo M. Structure–property correla-tion in oxide-ion and proton conductors for clean energy applications: recent experimental and computational ad-vancements. J Mater Chem A. 2022;10:5082–5110. doi:10.1039/d1ta10326a
Ashish K, Ajay K, Venkata K. Perovskite oxide based materi-als for energy and environment- oriented photocatalysis. ACS Catal. 2020;10(17):10253–10315. doi:10.1021/acscatal.0c02947
Tatsumi I. Inorganic perovskite oxides. Springer Handbook of Electronic and Photonic Materials. Springer Internation-al Publishing: Germany; 2017. 1572 p.
Wan-Jian Y, Baicheng W, Jie G, Qingde S, Zhenzhu L, Yanfa Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ Sci. 2019;12:442–462. doi:10.1039/c8ee01574k
Medvedev D. Trends in research and development of pro-tonic ceramic electrolysis cells. Int J Hydrog Energy. 2019;44:26711–26740. doi:10.1016/j.ijhydene.2019.08.130
King G, Woodward PMJ. Cation ordering in perovskites. Mater Chem. 2010;20:5785–5796. doi:10.1039/B926757C
Animitsa I. Double perovskites with structure-disordered oxygen sublattice as high-temperature proton conductors. In Perovskites: Structure, Properties and Uses. Nova Sci-ence Publishers, Inc.: USA; 2010. p. 501–524.
Murugaraj P, Kreuer K, He T, Schober T, Maier J. High pro-ton conductivity in barium yttrium stannate Ba2YSnO5.5. Solid State Ion. 1997;98:1–6. doi:10.1016/S0167-2738(97)00102-1
Baliteau S, Mauvy F, Fourcade S, Grenier J. Investigation on double perovskite Ba4Ca2Ta2O11. Solid State Sci. 2009;11:1572–1575. doi:10.1016/j.solidstatesciences.2009.06.023
Jalarvo N, Haavik C, Kongshaug C, Norby P, Norby T. Con-ductivity and water uptake of Sr4(Sr2Nb2)O11·nH2O and Sr4(Sr2Ta2)O11·nH2O. Solid State Ion. 2009;180:1151–1156. doi:10.1016/j.ssi.2009.05.021
Gurudeo N, Dharmendra Y, Shail U. Ruddlesden–Popper phase A2BO4 oxides: Recent studies on structure, electrical, dielectric, and optical properties. J Adv Ceram. 2020;9(2):29–148. doi:10.1007/s40145-020-0365-x
Hayden AE, Lingling M, Ram S, Anthony KC. Layered double perovskites. Annu Rev Mater Res. 2021;51:1–33. doi:10.1146/annurev-matsci-092320-102133
Armstrong AR, Anderson PA. Synthesis and structure of a new layered niobium blue bronze: Rb2LaNb2O7. Inorg Chem. 1994;33(19):4366–4369. doi:10.1021/ic00097a026
Le Berre F, Crosnier-Lopez MP, Fourque, JL. Cationic order-ing in the new layered perovskite BaSrTa2O7. Solid State Sci. 2004;6(1):53–59. doi:10.1016/j.solidstatesciences.2003.10.008
Fop S, McCombie K, Wildman E, Skakle J, Irvine J, Connor P, Savaniu C, Ritter C, Mclaughlin A. High oxide ion and pro-ton conductivity in a disordered hexagonal perovskite. Nat Mater. 2020;19:752–757. doi:10.1038/s41563-020-0629-4
Fop S, Dawson J, Fortes A, Ritter C, McLaughlin A. Hydra-tion and ionic conduction mechanisms of hexagonal perov-skite derivatives. Chem Mater. 2021;33:4651–4660. doi:10.1021/acs.chemmater.1c01141
Murakami T, Hester J, Yashima M. High proton conductivity in Ba5Er2Al2ZrO13, a hexagonal perovskite-related oxide with intrinsically oxygen-deficient layers. J Am Chem Soc. 2020;142:11653–11657. doi:10.1021/jacs.0c02403
Andreev R, Korona D, Anokhina I, Animitsa I. Proton and oxygen-ion conductivities of hexagonal perovskite Ba5In2Al2ZrO13. Mater. 2022;15(11):3944. doi:10.3390/ma15113944
Shpanchenko R, Abakumov A, Antipov E, Kovba L. Crystal structure of Ba5In2Al2ZrO13. J Alloy Compd. 1994;206:185–188. doi:10.1016/0925-8388(94)90033-7
Yashima M, Tsujiguchi T, Sakuda Y, Yasui Y, Zhou Y, Fujii K, Torii Sh, Kamiyama T, Skinner SJ. High oxide-ion con-ductivity through the interstitial oxygen site in Ba7Nb4MoO20-based hexagonal perovskite related oxides. Nat Commun. 2021;12(1):1–7. doi:10.1038/s41467-020-20859-w
Shannon R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogeni-des. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gen Crystallogr. 1976;32:751–767. doi:10.1107/S0567739476001551
Stotz S, Wagner C. Die Loslichkeit von Wasserdampf und Wasserstoff in Festen Oxiden. Ber Bunsenges Phys Chem. 1967;70(8):781–788. doi:10.1002/bbpc.19660700804
Lasia A. Electrochemical Impedance Spectroscopy and Its Applications. Modern Aspects of Electrochemistry. Spring-er: New York, USA; 2014. p. 143–258.
Irvine J, Sinclair D, West A. Electroceramics: Characteriza-tion by Impedance Spectroscopy. Adv Mater. 1990;2:132–138. doi:10.1002/adma.19900020304
Tarasova N, Animitsa I. Аnionic doping (F−, Cl−) as the method for improving transport properties of proton-conducting perovskites based on Ba2CaNbO5.5. Solid State Ion. 2018;317:21–25. doi:10.1016/j.ssi.2018.01.001
DOI: https://doi.org/10.15826/chimtech.2022.9.4.14
Copyright (c) 2022 Roman D. Andreev, Daniil V. Korona, Irina A. Anokhina, Irina E. Animitsa

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







