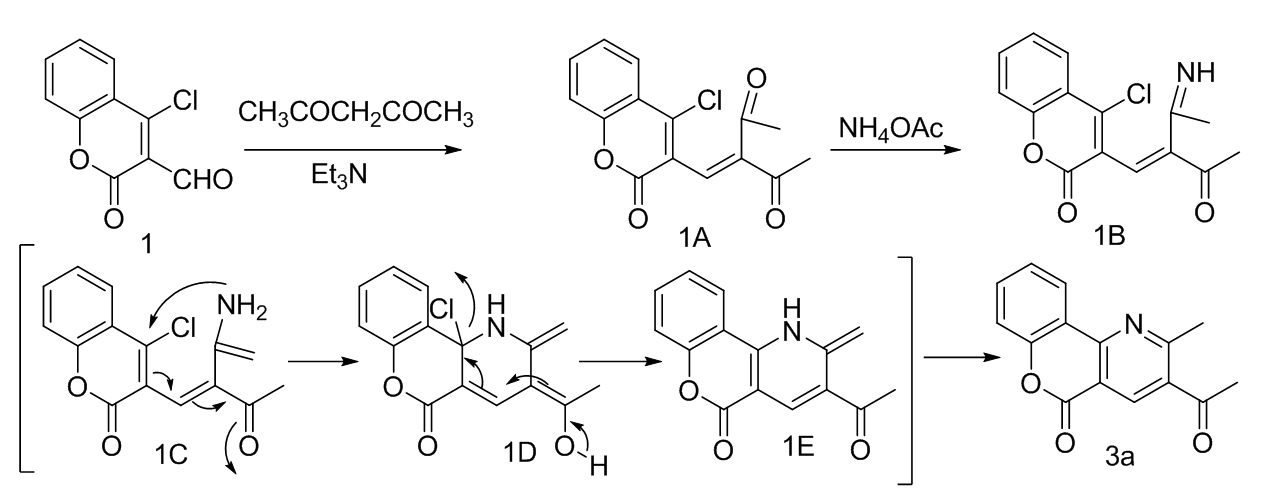
Synthesis of 5H-Chromeno[4,3-b]pyridin-5-one derivatives as a backbone of natural product polyneomarline C scaffolds in presence of Et3N and NH4OAc in EtOH
Abstract
Keywords
Full Text:
PDFReferences
Sarkar A, Santra S, Kundu SK, Hajra A, Zyryanov GV, Chupakhin ON, Charushin VN, Majee A. A decade update on solvent and catalyst-free neat organic reactions: a step forward towards sustainability. Green Chem. 2016;18:4475–4525. doi:10.1039/c6gc01279e
Egorov IN, Santra S, Kopchuk DS, Kovalev IS, Zyryanov GV, Majee A, Ranu BC, Rusinov VL, Chupakhin ON. Ball mill-ing: an efficient and green approach for asymmetric organic syntheses. Green Chem. 2020;22:302–315. doi:10.1039/C9GC03414E
Rahman M, Mukherjee A, Kovalev IS, Kopchuk DS, Zyryanov GV, Tsurkan MV, Majee A, Ranu BC, Charushin VN, Chupakhin ON, Santra S. Recent advances on diverse decarboxylative reactions of amino acids. Adv Synth Catal. 2019;361:2161–2214. doi:10.1002/adsc.201801331
Medina FG, Marrero JG, Macias-Alonso M, Gonzalez MC, Cordova-Guerrero I, Garcia AGT, Osegueda-Roblesa S. Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat Prod Rep. 2015;32:1472–1507. doi:10.1039/c4np00162a
Patra P, Kar GK, Sarkar A, Ray JK, Dasgupta T, Ghosh M, Bhattacharya S. N-Aryl Modification in γ-Lactam: Design and Synthesis of Novel Monocyclic γ-Lactam Derivatives as Inhibitor for Bacterial Propagation. Synth Commun. 2012;42:3031–3041. doi:10.1080/00397911.2011.574807
Mukherjee A, Mahato S, Zyryanov GV, Majee A, Santra S. Diverse synthesis of pyrano[3,2-c]coumarins: a brief update. New J Chem. 2020;44:18980–18993. doi:10.1039/D0NJ03846F
Sarkar S, Chatterjee R, Mukherjee A, Mukherjee D, Mandal NC, Mahato S, Santra S, Zyryanov GV, Majee A. Mechano-chemical synthesis and antimicrobial studies of 4-hydroxy-3-thiomethylcoumarins using imidazolium zwitterionic molten salt as an organocatalyst. ACS Sus Chem Eng. 2021;9:5557–5569. doi:10.1021/acssuschemeng.0c08975
Andersen RJ, Faulkner DJ, He C, Van Duyne GD, Clardy J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J Am Chem Soc. 1985;107:5492-5. doi:10.1021/ja00305a027.
Fan H, Peng J, Hamann MT, Hu JF. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem Rev. 2008;108:264–287. doi:10.1021/cr078199m
Ukawa K, Ishiguro T, Wada Y, Nohara A. Synthesis of 5-Oxo-5H-[1]benzopyrano[4,3-b]pyridine Derivatives. Heterocycles 1986;24:1931–1941. doi:10.3987/R-1986-07-1931
Heber D. Reaktionen an Heterocyclen mit 2-Acyl-2-propenon-Teilstruktur, 3. Mitt. Pyrido[3,2-c]cumarine aus 3-substituierten 1-Benzopyranen und Enaminen. Arch Pharm. 1987;320:402–406. doi:10.1002/ardp.19873200505
Heber D, Berghaus T. Synthesis of 5H-[1]benzopyrano[4,3-b]pyridin-5-ones containing an azacannabinoidal structure. J Heterocycl Chem. 1994;31:1353–1359. doi:10.1002/jhet.5570310610
Valencia E, Patra A, Freyer AJ, Shamma M, Fajardo V. Santi-agonamine: a new aporphinoid alkaloid incorporating a phenanthridine skeleton. Tetrahedron Lett. 1984;25:3163–3166. doi:10.1016/S0040-4039(01)90998-0
Lewis WH, Stonard RJ, Porras-Reyes B, Mustoe TA, (Walter H. Lewis, St. Louis, Mo, US), Int. PCT Pub. No WO/1992/ US00556847 A, 1992.
Levrier C, Balastrier M, Beattie KD, Carroll AR, Martin F, Choomuenwai V, Davis RA. Pyridocoumarin, aristolactam and aporphine alkaloids from the Australian rainforest plant Goniothalamus australis. Phytochem. 2013;86:121–126. doi:10.1016/j.phytochem.2012.09.019
Lu ZM, Zhang QJ, Chen RY, Yu DQ. Four new alkaloids from Polyalthia nemoralis. J Asian Nat Prod Res. 2008;10:656–664. doi:10.1080/10286020802242281
Balasubramanian M, Keay JG. In Comprehensive Heterocyclic Chemistry II; Katritzky AR, Rees CW, Scriven EFV, Eds.; Pergamon Press: Oxford; 1996. 245–300.
Chen J, Liu W, Ma J, Xu H, Wu J, Tang X, Fan Z, Wang PJ. Synthesis and Properties of Fluorescence Dyes: Tetracyclic Pyrazolo[3,4-b]Pyridine-Based Coumarin Chromophores with Intramolecular Charge Transfer Character. J Org Chem. 2012;77:3475–3482. doi:10.1021/jo3002722
Khan IA, Kulkarni MV, Gopal M, Shahabuddin MS, Sun CM. Synthesis and biological evaluation of novel angularly fused polycyclic coumarins. Bioorg Med Chem Lett. 2005;15:3584–3587. doi:10.1016/j.bmcl.2005.05.063
Patra P. A Concise Review on Pyridocoumarin/Azacoumarin Derivatives: Synthesis and Biological Activity. Chem Sel. 2019;4:2024–2043. doi:10.1002/slct.201803596
Ahn S, Yoon JA, Han YT. Total Synthesis of the Natural Pyr-idocoumarins Goniothaline A and B. Synth. 2019;51:552–556. doi:10.1055/s-0037-1610909
Patra P, Kar GK, Khatua B. Thermolysis of N-Aryl Enaminoimine Hydrochloride Derivatives: A Short and General Method for the Synthesis of Pyranoquinolin-3-one and Py-ranoacridin-3-one Derivatives. J Heterocycl Chem. 2014;51:1306–1310. doi:10.1002/jhet.1703
Patra P. Thermolysis of Chlorovinyl Imines as an Alternate Route for the Synthesis of Pyranoquinolin-3-one and Pyranoacridin-3-one Derivatives. J Heterocycl Chem. 2017;54:3656–662. doi:10.1002/jhet.2993
Sharapov AD, Fatykhov RF, Khalymbadzha IA, Sharutin VV, Santra S, Zyryanov GV, Chupakhin ON, Ranu BC. Mechanochemical synthesis of coumarins via Pechmann condensation under solvent-free conditions: An easy access to coumarins and annulated pyrano[2,3-f] and [3,2-f]indoles. Green Chem. 2022;24:2429–2437. doi:10.1039/D1GC04564D
Gurumurthy C, Fatima N, Reddy GN, Kumar CG, Sabitha G, Ramakrishna KVS. A diastereoselective synthesis of tetra-hydro- and dihydro-pyrido[2,3-c]coumarin derivatives via a one-pot three-component Povarov reaction catalyzed by bismuth(III) chloride. Bioorg Med Chem Lett. 2016;16:5119–5125. doi:10.1016/j.bmcl.2016.08.017
Chen Z, Hu L, Peng F. Efficient Synthesis of Functionalized Pyrido[2,3-c]coumarin Derivatives by a One-Pot Three-Component Reaction. Synlett. 2016;12:1888–1892. doi:10.1055/s-0035-1561610
Chatterjee R, Mukherjee A, Santra S, Zyryanov GV, Chupakhin ON, Majee A. An expedient solvent-free C-benzylation of 4-hydroxycoumarin with styrenes. Medeleev Commun. 2021;31:123–124. doi:10.1016/j.mencom.2021.01.039
Takano H, Narumi T, Nomura W, Tamamura H. Microwave-Assisted Synthesis of Azacoumarin Fluorophores and the Fluorescence Characterization. J Org Chem. 2017;82:2739–2744. doi:10.1021/acs.joc.6b02656
Gomha SM, Riyadh SM. Multicomponent Synthesis of Novel Penta-Heterocyclic Ring Systems Incorporating a Benzopyranopyridine Scaffold. Synth. 2014;46:258–262. doi:10.1055/s-0033-1340085
Keskin S, Balci M. Intramolecular Heterocyclization of O-Propargylated Aromatic Hydroxyaldehydes as an Expedient Route to Substituted Chromenopyridines under Metal-Free Conditions. Org Lett. 2015;17:964–967. doi:10.1021/acs.orglett.5b00067
Kornev MY, Tishin DS, Sosnovskikh VY. Reactions of chro-mone-3-carboxamides with 2-cyanothioacetamides. Mendeleev Commun. 2019;29:67–68. doi:10.1016/j.mencom.2019.01.022
Yadav A, Biswas S, Mobina SM, Samanta S. Efficient Cu(OTf)2-catalyzed and microwave-assisted rapid synthesis of 3,4-fused chromenopyridinones under neat conditions. Tettrahedron Lett. 2017;58:3634–3639. doi:10.1016/j.tetlet.2017.08.006
Al-Ayed AS. Synthesis of New Substituted Chromen[4,3-c]pyrazol-4-ones and Their Antioxidant Activities. Mol. 2011;16:10292–10302. doi:10.3390/molecules161210292
DOI: https://doi.org/10.15826/chimtech.2022.9.2.11
Copyright (c) 2022 Shrishnu Kumar Kundu, Susanta Patra, Chayan Sardar, Sunil Kumar Bhanja, Prasanta Patra

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






