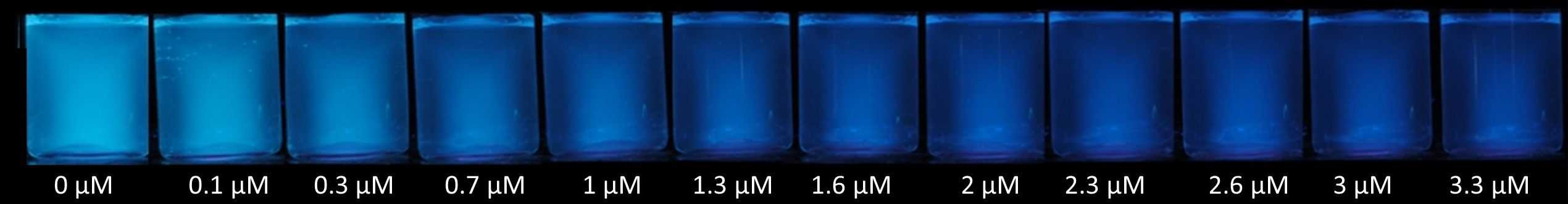
Bispyrenylalkane Chemosensor for the Naked-eye Detection of Nitro-explosives
Abstract
Keywords
Full Text:
PDFReferences
Yinon J, Zitrin S. The Analysis of Explosives. Elsevier; 1981. 322 p.
Kangas MJ, Burks RM, Atwater J, Lukowicz RM, Williams P, Holmes AE. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit Rev Anal Chem. 2017;47(2):138–53. doi:10.1080/10408347.2016.1233805
Jenkins TF, Walsh ME. Development of field screening methods for TNT, 2,4-DNT and RDX in soil. Talanta. 1992;39(4):419–28. doi:10.1016/0039-9140(92)80158-A
Li Z, Askim JR, Suslick KS. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem Rev. 2019;119(1):231–92. doi:10.1021/acs.chemrev.8b00226
Wen P, Amin M, Herzog WD, Kunz RR. Key challenges and prospects for optical standoff trace detection of explosives. TrAC - Trends Anal Chem. 2018;100:136–44. doi:10.1016/j.trac.2017.12.014
Sun X, Lei Y. Fluorescent carbon dots and their sensing applications. TrAC - Trends Anal Chem. 2017;89:163–80. doi:10.1016/j.trac.2017.02.001
Sun X, Wang Y, Lei Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem Soc Rev. 2015 Nov 21;44(22):8019–61. doi:10.1039/c5cs00496a
Zyryanov GV, Kopchuk DS, Kovalev IS, Nosova EV, Rusinov VL, Chupakhin ON. Chemosensors for detection of nitroaromatic compounds (explosives). Russ Chem Rev. 2014;83(9):783–819. doi:10.1070/RC2014v083n09ABEH004467
Salinas Y, Martínez-Máñez R, Marcos MD, Sancenón F, Costero AM, Parra M, Gil S. Optical chemosensors and reagents to detect explosives. Chem Soc Rev. 2012;41(3):1261–96. doi:10.1039/c1cs15173h
Zyryanov GV, Palacios MA, Anzenbacher P. Simple Molecule-Based Fluorescent Sensors for Vapor Detection of TNT. Org Lett. 2008;10(17):3681–4. doi:10.1021/ol801030u
Beyazkilic P, Yildirim A, Bayindir M. Formation of Pyrene Excimers in Mesoporous Ormosil Thin Films for Visual Detection of Nitro-explosives. ACS Appl Mater Interfaces. 2014;6(7):4997–5004. doi:10.1021/am406035v
Xiao FN, Wang K, Wang FB, Xia XH. Highly Stable and Luminescent Layered Hybrid Materials for Sensitive Detection of TNT Explosives. Anal Chem. 2015;87(8):4530–7. doi:10.1021/acs.analchem.5b00630
Demirel GB, Daglar B, Bayindir M. Extremely fast and highly selective detection of nitroaromatic explosive vapours using fluorescent polymer thin films. Chem Commun. 2013;49(55):6140–2. doi:10.1039/c3cc43202e
Andrew TL, Swager TM. A Fluorescence Turn-On Mechanism to Detect High Explosives RDX and PETN. J Am Chem Soc. 2007;129(23):7254–5. doi:10.1021/ja071911c
Mosca L, Karimi Behzad S, Anzenbacher P. Small-Molecule Turn-On Fluorescent Probes for RDX. J Am Chem Soc. 2015;137(25):7967–9. doi:10.1021/jacs.5b04643
Wu D, Sedgwick AC, Gunnlaugsson T, Akkaya EU, Yoon J, James TD. Fluorescent chemosensors: The past, present and future. Chem Soc Rev. 2017;46(23):7105–23. doi:10.1039/c7cs00240h
Ohno K, Satoh H, Iwamoto T. Quantum chemical exploration of dimeric forms of polycyclic aromatic hydrocarbons, naphthalene, perylene, and coronene. Chem Phys Lett. 2019;716:147–54. doi:10.1016/J.CPLETT.2018.12.034
Marsh AV, Cheetham NJ, Little M, Dyson M, White AJP, Beavis P, Warriner CN, Swain AC, Stavrinou PN, Heeney M. Carborane-Induced Excimer Emission of Severely Twisted Bis-o-Carboranyl Chrysene. Angew Chemie Int Ed. 2018;57(33):10640–5. doi:10.1002/anie.201805967
Šoustek P, Michl M, Almonasy N, Machalický O, Dvořák M, Lyčka A. The synthesis and fluorescence of N-substituted 1- and 2-aminopyrenes. Dye Pigment. 2008;78(2):139–47. doi:10.1016/j.dyepig.2007.11.003
Suzuki Y, Morozumi T, Nakamura H, Shimomura M, Hayashita T, Bartsh RA. New fluorimetric alkali and alkaline earth metal cation sensors based on noncyclic crown ethers by means of intramolecular excimer formation of pyrene. J Phys Chem B. 1998;102(40):7910–7. doi:10.1021/jp981567t
Hrdlovič P, Horinová L, Chmela Š. Spectral properties of ionic derivatives of pyrene and their aggregates with anionic surfactant and polyelectrolyte. Can J Chem. 1995;73(11):1948–54. doi:10.1139/v95-240
Daems D, Van den Zegel M, Boens N, De Schryver FC. Fluorescence decay of pyrene in small and large unilamellar L,α-Dipalmitoylphosphatidylcholine vesicles above and below the phase transition temperature. Eur Biophys J. 1985;12(2):97–105. doi:10.1007/BF00260432
Kim JJ, Beardslee RA, Phillips DT, Offen HW. Fluorescence lifetimes of pyrene monomer and excimer at high pressures. J Chem Phys. 1969;51:2761–2. doi:10.1063/1.1672406
Ruiu A, Vonlanthen M, Rojas-Montoya SM, González-Méndez I, Rivera E. Unusual fluorescence behavior of pyrene-amine containing dendrimers. Molecules. 2019;24(22). doi:10.3390/molecules24224083
Lin TI. Excimer fluorescence of pyrene-tropomyosin adducts. Biophys Chem. 1982;15(4):277–88. doi:10.1016/0301-4622(82)80011-2
Wang X, Liu L, Zhu S, Peng J, Li L. Preparation of exciplex-based fluorescent organic nanoparticles and their application in cell imaging. RSC Adv. 2017;7(65):40842–8. doi:10.1039/c7ra08142a
Kanagalingam S, Spartalis J, Cao TM, Duhamel J. Scaling relations related to the kinetics of excimer formation between pyrene groups attached onto poly(N,N-dimethylacrylamide)s. Macromolecules. 2002;35(22):8571–7. doi:10.1021/ma020784w
Bertolotti SG, Previtali CM. Fluorescence of pyrene derivatives in the presence of poly(methallyl sulfonate-vinyl acetate) copolymers. effect of charge density. J Macromol Sci Part A. 1994;31(4):439–49. doi:10.1080/10601329409351530
Förster T, Kasper K. Ein Konzentrationsumschlag der Fluoreszenz des Pyrens. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für Phys Chemie. 1955;59(10):976–80. German. doi:10.1524/zpch.1954.1.5_6.275
Rehm D, Weller A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr J Chem. 1970;8(2):259–71. doi:10.1002/ijch.197000029
Goodpaster JV, McGuffin VL. Fluorescence quenching as an indirect detection method for nitrated explosives. Anal Chem. 2001;73(9):2004–11. doi:10.1021/ac001347n
Zachariasse K, Kühnle W. Intramolecular Excimers with α,ω-Diarylalkanes. Zeitschrift für Phys Chemie. 1976;101(1–6):267–76. doi:10.1524/zpch.1976.101.1-6.267
Ikeda T, Lee B, Tazuke S, Takenaka A. Time-resolved observation of excitation hopping between two anthryl moieties attached to both ends of alkanes: simulation based on conformational analysis. J Am Chem Soc. 1990;112(12):4650–6. doi:10.1021/ja00168a004
Zhang P, Zhang L, Wang H, Zhang DW, Li ZT. Helical folding of an arylamide polymer in water and organic solvents of varying polarity. Polym Chem. 2015;6(15):2955–61. doi:10.1039/C5PY00096C
Ikai T, Shimizu S, Awata S, Kudo T, Yamada T, Maeda K, Kanoh S. Synthesis and chiroptical properties of a π-conjugated polymer containing glucose-linked biphenyl units in the main chain capable of folding into a helical conformation. Polym Chem. 2016;7(48):7522–9. doi:10.1039/C6PY01759B
Shrivastava A, Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011;2(1):21. doi:10.4103/2229-5186.79345
DOI: https://doi.org/10.15826/chimtech.2021.8.2.09
Copyright (c) 2021 I.S. Kovalev, L.K. Sadieva, O.S. Taniya, V.M. Yurk, A.S. Minin, D.S. Kopchuk, G.V. Zyryanov, V.N. Charushin, O.N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






