Synthesis of meso-2,2’-bipyridyl-substituted calix[4]arenes and their response to metal cations
Abstract
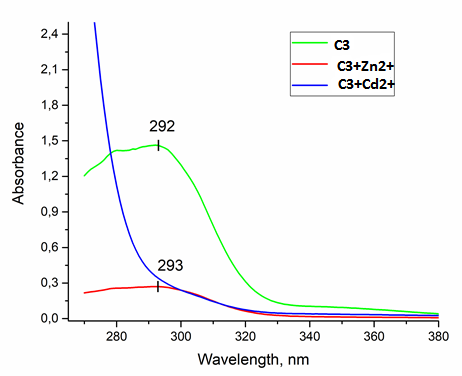
A convenient synthetic approach to meso-substituted with 2,2’-bipyridine and 1-(pyridin-2-yl)isoquinoline residues calix[4]arenes is reported. This approach involves the reaction of generated in situ 2-lithio-calix[4]arene with 1,2,4-triazine precursor with the following aromatization of the obtained adduct, and the aza-Diels-Alder reaction of the 1,2,4-triazinyl-substituted calix[4]arene with 2,5-norbornadien or in-situ generated 1,2-dehydrobenzene. The UV/fluorescence response of thus obtained meso-pyridyl-substituted calix[4]arenes to metal cations is studied.
Keywords
Full Text:
PDFReferences
Ludwig R. Calixarenes in analytical and separation chemistry. Fresenius J Anal Chem. 2000;367:103–28. doi:10.1007/s002160051611
Diamond D, Nolan K. Calixarenes: Designer Ligands for Chemical Sensors. Anal Chem. 2001;73:22A–29A. doi:10.1021/ac012376g
Kumar R, Sharma A, Singh H, Suating P, Kim HS, Sunwoo K, Shim I, Gibb BC, Kim JS. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem Rev. 2019;119:9657–721. doi:10.1021/acs.chemrev.8b00605
Veber F, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23. doi:10.1021/jm020017n
Dumazet-Bonnamour I, Halouani H, Oueslati F, Lamartine R. Calixarenes for metal cations extraction. C R Chim. 2005;8:881–91. doi:10.1016/j.crci.2005.02.004
Abraham W. Inclusion of organic cations by calix[n]arenes. J Incl Phenom Macro. 2002;43:159–74. doi:10.1023/A:1021288303104
Staffilani M, Hancock KSB, Steed JW, Holman KT, Atwood JL, Juneja RK, Burkhalter RS. Anion Binding within the Cavity of π-Metalated Calixarenes. J Am Chem Soc. 1997;119:6324–35. doi:10.1021/ja9702172
Atwood JL, Barbour LJ, Heaven MW, Raston CL. Association and orientation of C70 on complexation with calix[5]arene. Chem Commun. 2003;3:2270–1. doi:10.1039/B306411P
Varaksin MV, Utepova IA, Chupakhin ON, Charushin VN. Synthesis of New meso-Substituted Heterocyclic Calix[4]arenes via S-N(H) Approach. Macroheterocycles. 2013;6:308–14. doi:10.6060/mhc131268c
Dalbavie JO, Regnouf-de-Vains JB, Lamartine R, Perrin M, Lecocq S, Fenet B. A Calix[4]arene-Based Bipyridine Podand as Versatile Ligand for Transition Metal Cations. Eur J Inorg Chem. 2002;4:901–9. doi:10.1002/1099-0682(200203)2002:4<901::AID-EJIC901>3.0.CO;2-9
Beer PD, Szemes F, Passaniti P, Maestri M. Luminescent Ruthenium(II) Bipyridine−Calix[4]arene Complexes as Receptors for Lanthanide Cations. Inorg Chem. 2004;43:3965–75. doi:10.1021/ic0499401
Dorta R, Shimon LJW, Rozenberg H, Ben-David Y, Milstein D. A New Ligand System Based on a Bipyridine-Functionalized Calix[4]arene Backbone Leading to Mono- and Bimetallic Complexes. Inorg Chem. 2003;42:3160–7. doi:10.1021/ic020643a
Beer PD, Martin JP, Drew MGB. Calix[4]arene cryptand and new 1,3-bis-pyridyl,-bipyridyl and -alkylthioether calix[4]arenes designed to coordinate transition metal cations. Tetrahedron. 1992;48:9917–28. doi:10.1016/S0040-4020(01)92282-3
Regnouf-de-Vains JR, Lamartine R. Synthesis and Complexation Properties of a New Class of Receptors based on a cone‐configurated tetra‐p‐(tert‐butyl)calix[4]arene and bipyridyl subunits. Helv Chim Acta. 1994;77:1817–25. doi:10.1002/hlca.19940770713
Grigg R, Holmes JM, Jones SK, Norbert WDAJ. Luminescent pH sensors based on p-tert-butylcalix[4]arene-linked ruthenium(II) trisbipyridyl complexes. J Chem Soc, Chem Commun. 1994;2:185–7. doi:10.1039/C39940000185
Ulrich G, Ziessel R. Calixarene[4]-podands and barrel-shaped calixarene[4]-cryptands based on 5,5′-substituted-2,2′-bipyridine subunits. Tetrahedron Lett. 1994;35:6299–302. doi:10.1016/S0040-4039(00)73416-2
Regnouf-De-Vains JB, Lamartine R, Fenet B. Electrospray mass spectrometric evidence of calixarene p-quinone methide formation. Helv Chim Acta. 1998;81:661–9. doi:10.1002/(SICI)1096-9888(1998100)33:10<968::AID-JMS706>3.0.CO;2-M
Dalbavie JO, Regnouf-de-Vains JB, Lamartine R, Lecocq S, Perrin M. Complexation of cobalt(II) at the upper rim of two new calix[4]arene/bipyridine-based podands. Eur J Inorg Chem. 2000;4:683–91. doi:10.1002/(SICI)1099-0682(200004)2000:4<683::AID-EJIC683>3.0.CO;2-N
Beresnev DG, Itsikson NA, Chupakhin ON, Charushin VN, Kodess MI, Butakov AI, Rusinov GL, Morzherin YY, Konovalov AI, Antipin IS. One-step heterylation at the upper rim of calix[4]arene with 1,2,4-triazin-5(2H)-ones. J Org Chem. 2006;71:8272–5. doi:10.1021/jo061069d
(a) Pabst GR, Sauer J. A new and simple 'LEGO' system for the synthesis of 2,6-oligopyridines. Tetrahedron Lett. 1998;39:6687–90. doi:10.1016/S0040-4039(98)01437-3; (b) Pabst GR, Schmid K, Sauer J. A new and simple 'LEGO' system for the synthesis of branched oligopyridines. Tetrahedron Lett. 1998;39:6691–4. doi:10.1016/S0040-4039(98)01438-5; (c) Pabst GR, Sauer J. The new and simple 'LEGO' system: Its application to the synthesis of superbranched oligopyridines. Tetrahedron Lett. 1998;39:8817–20. doi:10.1016/S0040-4039(98)02042-5; (d) Pfüller OC, Sauer J. The new and simple ‘LEGO’ system for the synthesis of thienyl substituted 2,6-oligopyridines. Tetrahedron Lett. 1998;39:8821–4. doi:10.1016/S0040-4039(98)02043-7; (e) Pabst GR, Pfüller OC, Sauer J. The new and simple 'LEGO' system: Its application for the synthesis of 6-oligopyridyl-1,5,12-triazatriphenylenes. Tetrahedron Lett. 1998;39:8825–8. doi:10.1016/S0040-4039(98)02044-9; (f) Pabst GR, Pfüller OC, Sauer J. The new and simple 'LEGO' System: Synthesis and reactions of ruthenium(II) complexes. Tetrahedron. 1999;55:8045–64. doi:10.1016/S0040-4020(99)00422-6; (g) Stanforth SP, Tarbit B, Watson MD. Synthesis of pyridine and 2,2′-bipyridine derivatives from the aza Diels–Alder reaction of substituted 1,2,4-triazines. Tetrahedron. 2004;60:8893–7. doi:10.1016/j.tet.2004.07.024
(a) Rykowski A, Branowska D, Kielak J. A novel one-pot synthesis of annulated 2,2'-bipyridine ligands by inverse electron demand Diels-Alder reaction of 5,5'-bi-1,2,4-triazines. Tetrahedron Lett. 2000;41:3657–9. doi:10.1016/S0040-4039(00)00436-6; (b) Branowska D, Rykowski A. Application of 1-vinylimidazole in Diels-Alder reaction of 5,5′-bi-1,2,4-triazines. Synlett. 2002:1892–4. doi:10.1055/s-2002-34880; (c) Branowska D, Kielak J. A facile synthesis of annulated 2,2′-bipyridine ligands with alkylsulfanyl and alkylsulfonyl substituents in the 6 and 6' positions. Pol J Chem. 2003;77:1149–55. (d) Kozhevnikov DN, Kozhevnikov VN, Nikitina TV, Rusinov VL, Chupakhin ON, Neunhoeffer H. Synthesis of functionalised bipyridines by sequential nucleophilic substitution of hydrogen and cycloaddition in 1,2,4-triazine rings. Mendeleev Commun. 2002;12:30–2. doi:10.1070/MC2002v012n01ABEH001548
Raw SA., Taylor RJK. Highly substituted pyridines via tethered imine–enamine (TIE) methodology. Chem Commun. 2004;5:508–9. doi:10.1039/B316107B
(a) Kopchuk DS, Kovalev IS, Khasanov AF, Zyryanov GV, Slepukhin PA, Rusinov VL, Chupakhin ON. A rational protocol for the synthesis of 1-(2-pyridyl)isoquinolines. Mendeleev Commun. 2013;23:142–4. doi:10.1016/j.mencom.2013.05.007; (b) Kopchuk DS, Nikonov IL, Zyryanov GV, Kovalev IS, Rusinov VL, Chupakhin ON. Preparation of 3-cyano-1-(2-pyridyl)isoquinolines by using aryne intermediates. Chem Heterocycl Compd. 2014;50:907–10. doi:10.1007/s10593-014-1545-9; (c) Kopchuk DS, Nikonov IL, Khasanov AF, Giri K, Santra S, Kovalev IS, Nosova EV, Gundala S, Venkatapuram P, Zyryanov GV, Majee A, Chupakhin ON. Studies on the interactions of 5-R-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels-Alder reaction versus aryne-mediated domino process. Org Biomol Chem. 2018;16:5119–31. doi:10.1039/C8OB00847G
DOI: https://doi.org/10.15826/chimtech.2020.7.4.14
Copyright (c) 2020 Moseev T.D., Khasanov A.F., Varaksin M.V., Kopchuk D.S., Kovalev I.S., Taniya O.S., Rahman M., Santra S., Zyryanov G.V., Chupakhin O.N., Charushin V.N.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






