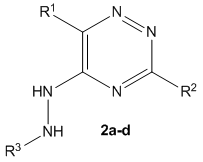
A convenient synthetic approach to 5-(het)arylhydrazine substituted 1,2,4-triazines
Abstract
A convenient synthesis of 1,2,4-triazines bearing the moieties of (hetero)arylhydrazines at the position of C5 of the 1,2,4-triazine core is reported.
Keywords
Full Text:
PDFReferences
Al-Matar HM, Khalil KD, Al-Dorri DM, Elnagdi MH. Efficient routes to pyrazolo[3,4-e][1,2,4]triazines and a new ring system: [1,2,4]triazino[5,6-d][1,2,3]triazines. Molecules. 2010;15:3302–10. doi:10.3390/molecules15053302
Heunhoeffer H, Reichel D. Triazino[6,5-e[-1,2,4-triazine; II. Synthesis. 1988;11:877–9. doi:10.1055/s-1988-27734
Molina P, Tárraga A, Espinosa A, Lidón M.J. Oxamic Acid Derivatives in Heterocyclic Synthesis: Preparation of 1,4-Oxazine and 1,2,4-Triazino[5,6-e[[1,3,4]oxadiazine Derivatives. Synthesis. 1987;2:128–34. doi:10.1055/s-1987-27860
Sayed AR. Synthesis of 1,3,4-thiadiazines, bis-1,3,4-thiadiazoles, [1,2,4]triazino[3,4-b][1,3,4]thiadiazine, thiazolines from carbonothioic dihydrazide. Tetrahedron. 2012;68:2784–9. doi:10.1016/j.tet.2012.02.011
Juhász-Riedl Zs, Hajós Gy, Kollenz G, Messmer A. Synthesis and ring transformation of a new fused as triazinium salt. Chem Ber. 1989;122:1935–8. doi:10.1002/cber.19891221018
(a) Charushin VN, Chupakhin ON. Nucleophilic C-H functionalization of arenes: a contribution to green chemistry. Russ Chem Bull Int Ed. 2019;68:453–71. doi:10.1007/s11172-019-2441-3; (b) Chupakhin ON, Rusinov VL, Ulomsky EN, Kojevnikov DN, Neunhoeffer H. Nucleophilic substitution of hydrogen in the reaction of 1,2,4-triazine-4-oxides with cyanides. Mendeleev Commun. 1997;7:66–7. doi:10.1070/MC1997v007n02ABEH000700
Huang JJ. Synthesis of Fused 1,2,4-Triazines: 6-and 7-Azapteridine and 6-Azapurine Ring Systems. J Org Chem. 1985;50:2293–8. doi:10.1021/jo00213a019
Kozhevnikov DN, Kozhevnikov VN, Kovalev IS, Rusinov VL, Chupakhin ON, Aleksandrov GG. Transformations of 1,2,4-Triazines in Reactions with Nucleophiles: V. SNH and ipso-Substitution in the Synthesis and Transformations of 5-Cyano-1,2,4-triazines. Russ J Org Chem. 2002;38:744–50. doi:10.1023/A:1019631610505
Savchuk MI, Khasanov AF, Kopchuk DS, Krinochkin AP, Nikonov IL, Starnovskaya ES, Shtaitz YK, Kovalev IS, Zyryanov GV, Chupakhin ON. New push-pull fluorophores on the basis of 6-alkoxy-2,2'-bipyridines: rational synthetic approach and photophysical properties. Chem Heterocycl Compds. 2019;55:554–9. doi:10.1007/s10593-019-02495-5
Rusinov VL, Zyryanov GV, Egorov IN, Ulomskii EN, Aleksandrov GG, Chupakhin ON. Synthesis of 8-Aryl[1,2,4]triazolo[1,5-d][1,2,4]triazin-5(6H)-ones by SNH Reactions. Russ J Org Chem. 2004;40:85–9. doi:10.1023/B:RUJO.0000034914.87626.86
Rykowski A, Branowska D, Makosza M, Van LyP. Reactions of 1,2,4-Triazines with Nitromethide Ion. A Convenient Method of Preparation of 1,2,4-Triazin-5-ylcarbaldehyde Oximes and their Synthetic Applications. J Heterocycl Chem. 1996;33:1567–71. doi:10.1002/jhet.5570330603
Kopchuk DS, Chepchugov NV, Kovalev IS, Santra S, Rahman M, Giri K, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON. Solvent-free synthesis of 5-(aryl/alkyl)amino-1,2,4-triazines and α-arylamino-2,2′-bipyridines with greener prospects. RSC Adv. 2017;7:9610–9. doi:10.1039/C6RA26305D
Kopchuk DS, Starnovskaya ES, Shtaitz YK, Khasanov AF, Kim GA, Nosova EV, Krinochkin AP, Zyryanov GV, Rusinov VL, Chupakhin ON. 5-Aryl-2,2′-bipyridines bearing fluorinated anilines residues at C6 position: synthesis and photophysical properties. Res Chem Intermed. 2020;48:3929–44. doi:10.1007/s11164-020-04182-z
Starnovskaya ES, Savchuk MI, Shtaitz YaK, Kopchuk DS, Kovalev IS, Taniya OS, Pavlyuk DE, Khasanov AF, Zyryanov GV, Chupakhin ON. Polynuclear Aromatic Amines as N-Nucleophiles in the ipso-Substitution of the Cyano Group in 1,2,4-Triazines. Russ J Org Chem. 2020;56:335–8. doi:10.1134/S1070428020010268
Prokhorov AM, Kozhevnikov DN, Rusinov VL, Chupakhin ON, Glukhov IV, Antipin MY, Kazheva ON, Chekhlov AN, Dyachenko OA, Organometallics. 2006;25:2972–7. doi:10.1021/om051058v
Ohba S, Konno S, Yamanaka H. Studies on as-Triazine Derivatives: XVI: Reaction of 1,2,4-Triazinecarbonitriles with Carbanions. Chem Pharm Bull. 1991;39:486–8. doi:10.1248/cpb.39.486
Kozhevnikov DN, Kovalev IS, Prokhorov AM, Rusinov VL, Chupakhin ON. SNH reactions of pyrazine N-oxides and 1,2,4-triazine 4-oxides with CH-active compounds. Russ Chem Bull Int Ed. 2003;52:1588–94. doi:10.1023/A:1025601311393
Kopchuk DS, Krinochkin AP, Starnovskaya ES, Shtaitz YaK, Kovalev IS, Zyryanov GV, Rusinov VL, Chupakhin ON. Substitution of Cyano Group in Position 5 of 1,2,4-Triazines by Carboxylic Acid Hydrazide Residues under Solvent-Free Conditions. Russ J Org Chem. 2018;54:509–11. doi:10.1134/S1070428018030223
Krinochkin AP, Kopchuk DS, Giri K, Shtaitz YaK, Starnovskaya ES, Khalymbadzha IA, Drokin RA, Ulomsky EN, Santra S, Zyryanov GV, Rusinov VL, Chupakhin ON. A PASE Approach towards (Adamantyl-1)-, Alkyl- and (Het)Aryl-Substituted [1,2,4]triazolo[1,5-d][1,2,4]triazines: A Sequence of Two Solvent-Free Reactions Bearing Lower E-Factors. ChemistrySelect. 2018;3:8202–6. doi:10.1002/slct.201801244
Rosen BR, Werner EW, O'Brien AG, Baran PS. Total Synthesis of Dixiamycin B by Electrochemical Oxidation. J Am. Chem Soc. 2014;136:5571–4. doi:10.1021/ja5013323
Zhang Q, Mandi A, Li S, Chen Y, Zhang W, Tian X, Zhang H, Li H, Zhang W, Zhang S, Ju J, Kurtan, T, Zhang C. N–N‐Coupled Indolo‐sesquiterpene Atropo‐Diastereomers from a Marine‐Derived Actinomycete. Eur J Org Chem. 2012;27:5256−62. doi:10.1002/ejoc.201200599
Klug DM, Diaz-Gonzalez R, Perez-Moreno, G, Ceballos-Perez G, Garcıa-Hernandez R, GomezPerez V. Evaluation of a class of isatinoids identified from a high-throughput screen of human kinase inhibitors as anti-Sleeping Sickness agents. PLoS Negl Trop Dis. 2019;13(2):e0007129. doi:10.1371/journal.pntd.0007129
DOI: https://doi.org/10.15826/chimtech.2020.7.4.12
Copyright (c) 2020 Krinochkin A.P., Guda M.R., Rammohan A., Kopchuk D.S., Zyryanov G.V., Rusinov V.L., Chupakhin O.N.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






