Sheelite-related strontium molybdates: synthesis and characterization
Abstract
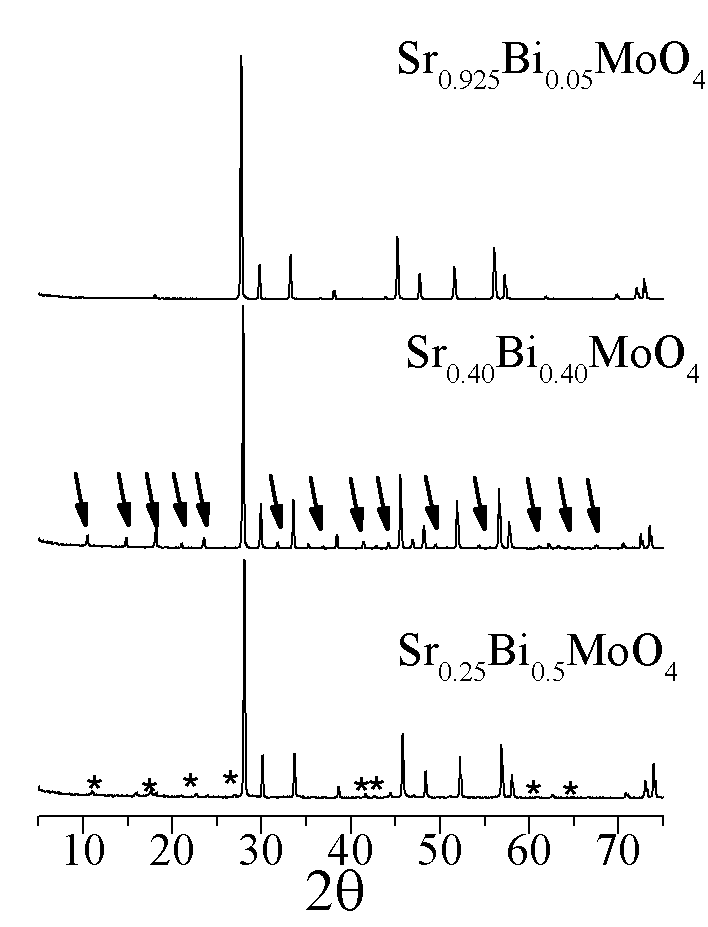
Sr1-1.5xBixMoO4, a superstructural ordering was observed. Conductivity and dielectric loss of ceramic samples are measured using alternating current.
Keywords
Full Text:
PDFReferences
Mikhailik VB, Kraus H, Miller G, Mykhaylyk MS, Wahl D. Luminescence of CaWO4, CaMoO4, and ZnWO4 scintillating crystals under different excitations. J. Appl. Phys. 2005:97(8):083523. doi:10.1063/1.1872198
Faure N, Borel C, Couchaud M, Basset G, Templier R, and Wyon C. Optical properties and laser performance of neodymium doped scheelites CaWO4 and NaGd(WO4)2. Appl. Phys. B: Lasers Opt. 1996:63(6):593–98. doi:10.1007/BF01830998
Sharma N, Shaju KM, Rao GVS, Chowdari BVR, Dong ZL, White TJ. Carbon-Coated Nanophase CaMoO4 as Anode Material for Li Ion Batteries. Chem. Mater. 2004:16 (3):504–12. doi:10.1021/cm0348287
Cavalcante LS, Longo VM, Sczancoski JC, Almeida MAP, Batista AA, Varela JA, Orlandi MO, Longo E, Liu MS. Electronic structure, growth mechanism and photoluminescence of CaWO4 crystal. Cryst. Eng. Comm. 2012:14(3):853–68. doi:10.1039/C1CE05977G
Yao WF, Ye JHJ. Photophysical and photocatalytic properties of Ca1-xBixVxMo1-xO4 solid solutions. Phys. Chem. B. 2006:110(23):11188–95 doi:10.1021/jp0608729
Choi GK., Kim JR., Yoon SH., Hong KS. Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A = Mg, Zn, Mn) AMoO4 compounds. J. Eur. Ceram. Soc. 2007:27(8-9):3063–67. doi:10.1016/j.jeurceramsoc.2006.11.037
Esaka T, Mina-ai T, Iwahara H. Oxide ion conduction in the solid solution based on the scheelite-type oxide PbWO4 // Solid State Ionics: 1992:57(3-4): 319–25. doi:10.1016/0167-2738(92)90165-L
Zhang GG., Fang QF., Wang XP., Yi ZG. Dielectric relaxation study of Pb1-xLaxMoO4+δ (x = 0–0.3) oxide-ion conductors. J. Phys.: Condens. Matter .2003:15(24): 4135–42. doi:10.1088/0953-8984/15/24/307
Cheng J, Liu C, Cao W, Qi M, Shao G. Synthesis and electrical properties of scheelite Ca1-xSmxMoO4+d solid electrolyte ceramics . Mat. Res. Bull. 2011:46(2):185–89. doi:10.1016/j.materresbull.2010.11.019
Md. Haque M., Kim D-K. Luminescent properties of Eu activated MLa2(MoO4)4 based (M=Ba, Sr and Ca) novel red-emitting phosphors. Mater. Lett. 2009:3(9-10):793-96. doi:10.1016/j.matlet.2009.01.018
Jiang P., Gao W., Cong R., Yang T. Structural investigation of the A-site vacancy in scheelites and the luminescence behavior of two continuous solid solutions A1-1.5xEux•0.5xWO4 and A0.64–0.5yEu0.24Liy•0.12–0.5yWO4 (A = Ca, Sr; • = vacancy). Dalton Trans. 2015:44(13):6175-83. doi:10.1039/c5dt00022j
Tomaszewicz E, Kaczmarek SM, Fuks H. New cadmium and rare-earth metal molybdates with scheelite type structure // Mater. Chem. Phys. 2010:122(2-3):595-601. doi:10.1016/j.matchemphys.2010.03.052
Su Y, Li L, Li G. Synthesis and optimum luminescence of CaWO4-based red phosphors with codoping of Eu3+ and Na+ . Chem. Mater. 2008:20(19):6060–67. doi:10.1021/cm8014435
Sameera S, Prabhakar Rao P, Divya S, Raj KV, Aju Thara TR. High IR reflecting BiVO4-CaMoO4 based yellow pigments for cool roof applications. Energ. Buildings. 2017:154: 491-98. doi:10.1016/j.enbuild.2017.08.089
Sleight AW, Aykan K, Rogers DB. New nonstoichiometric molybdate, tungstate, and vanadate catalysts with the scheelite-type structure. J. Solid State Chem. 1975:13(3): 231-36. doi:10.1016/0022-4596(75)90124-3
Guo J, Randall CA, Zhang G, Zhou D, Chen Y, Wang H. Synthesis, structure, and characterization of new low-firing microwave dielectric ceramics: Ca1−3xBi2xФxMoO4. J. Mater. Chem. C. 2014:2(35):7364-72. doi:10.1039/c4tc00698d
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67. doi:10.1107/S0567739476001551
Basiev TT, Sobol AA, Voronko YK, Zverev PG. Spontaneous Raman spectroscopy of tungstate and molybdate crystals for raman lasers. Opt. Mater.2000:15(3):205-16. doi:10.1016/S0925-3467(00)00037-9
Cho Y, Bull Y. Fine-tuning the emission color of a transparent suspension of SrMoO4: Eu3+,Tb3+ nanophosphors. Korean Chem. Soc. 2015:36(1):282–86. doi:10.1002/bkcs.10065
DOI: https://doi.org/10.15826/chimtech.2018.5.4.03
Copyright (c) 2018 Zoya Alekseevna Mikhaylovskaya, Elena Stanislavovna Buyanova, Sofia Aleksandrovna Petrova, Alena Andreevna Nikitina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






