Preparation, Structure and Physicochemical Properties of La0.95Bi0.05Mn1-yCuyO3+δ (у=0.1-0.4) and Composites with Bi2O3-Based Electrolytes
Abstract
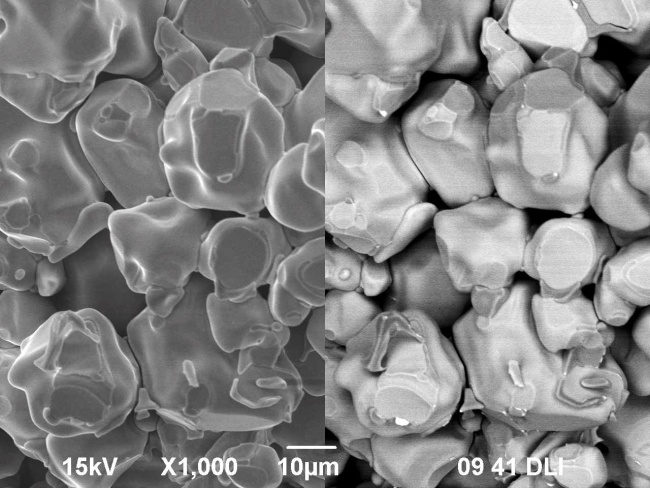
Samples of La0.95Bi0.05Mn1-yCuyO3+δ (у=0.1-0.4) were prepared by solid-state synthesis. Additionally, the sample with nominal composition La0.95Bi0.05Mn0.7Cu0.3O3+δ was obtained using citrate-nitrate method. It was determined by X-ray diffraction analysis that the compounds have rhombohedral (space group R-3c) or orthorhombic (space group Pbnm) structure, depending on the composition. Single-phase compounds are synthesized at у=0.1; 0.2. The investigation using scanning electron microscope showed that the grain sizes for the samples sintered using different techniques are close to each other because of the high calcination temperature. For the sample with orthorhombic structure the phase transition into rhombohedral one was found around 390 °С by means of dilatometry. Thermal expansion coefficient of the sample La0.95Bi0.05Mn0.7Cu0.3O3+δ is equal to 6·10-6 K-1 (T < 390 °C) and 15·10-6 K-1 (T > 390 °C). The composite materials of substituted lanthanum manganites with Bi4V1.7Fe0.3O11-δ and Bi7Nb1.8Zr0.2O15.5-δ solid electrolytes were obtained at 650 °C. The electrical conductivity values for the latter one are by three orders of magnitude higher than for pure bismuth niobate.
Keywords
Full Text:
PDFReferences
Kuo JH, Anderson HU, Sparlin DM. Oxidation-reduction behavior of undoped and Sr-doped LaMnO3. Defect structure, electrical conductivity, and thermoelectric power. J Solid State Chem. 1990;87:55–63. doi:10.1016/0022-4596(90)90064-5
Ruffa AR. Thermal expansion in insulating materials. Mater Sci. 1980;15:2258–67. doi:10.1007/BF00552315
Ghosh K, Ogale SB, Ramesh R. Transition-element doping effects in La0.7Ca0.3MnO3. Phys Rev. 1999;59:533-7. doi:10.1103/PhysRevB.59.533
Jia L, Gao J, Fang W. Influence of copper content on structural features and performance of pre-reduced LaMn1-xCuxO3 (0≤x<1) catalysts for methanol synthesis from CO2/H2. J Rare Earth. 2010;28:747–51. doi:10.1016/S1002-0721(09)60193-9
Gallagher PK, Johnson DW, Vogel EM. Preparation, structure, and selected catalytic properties of the system LaMn1-xCuxO3-y. J Am Ceram Soc. 1977;60:28–31. doi:10.1111/j.1151-2916.1977.tb16086.x
Porta P, De Rossi S, Faticanti M, Minelli G, Pettiti I, Lisi L, Turco M. Perovskite-type oxides I. Structural, magnetic and morphological properties of LaMn1-xCuxO3 and LaCo1-xCuxO3 solid solutions with large surface area. J Solid State Chem. 1999;146:291304. doi:10.1006/jssc.1999.8326
Rojas ML, Fierro JLG, Tejuca LG, Bell AT. Preparation and characterization of
LaMn1-хCuхO3+λ perovskite oxides. J Catal. 1990;124:41–51. doi:10.1016/0021-9517(90)90102-P
Chan KS, Ma J, Jaenicke S, Chuah GK, Lee JY. Catalytic carbon-monoxide oxidation over strontium, cerium and copper-substituted lanthanum manganates and cobaltates. Appl Catal A. 1994;107:201–7. doi:10.1016/0926-860X(94)85156-5
Brown Bourzutschky JA, Homs N, Bell AT. Conversion of synthesis gas over
LaMn1-хCuхO3+ perovskites and related copper catalysts. J Catal. 1990;124:52–72. doi:10.1016/0021-9517(90)90103-Q
Tabata K, Hirano Y, Suzuki E. XPS studies on the oxygen species of LaMn1xCuxO3+λ. Appl Catal A. 1998;170:245–54.
Adler SB. Factors Governing Oxygen Reduction in Solid oxide Fuel cell Cathodes. Chem Rev. 2004;104:4791-844. doi:10.1021/cr020724o
Jiang Z, Zhang L, Cai L, Xia C. Bismuth oxide-coated (La,Sr)MnO3 cathodes for intermediate temperature solid oxide fuel cells with ittria-stabilized zirconia electrolytes. Electrochim Acta. 2009;54:3059–65. doi:10.1016/j.electacta.2008.11.067
Jiang Z, Zhang L, Feng K, Xia C. Nanoscale bismuth oxide impregnated (La,Sr)MnO3 cathodes for intermediate-temperature solid oxide fuel cells. J Power Sources. 2008;85:40-8. doi:10.1016/j.jpowsour.2008.07.003
Kaymieva OS, Danilova VV, Morozova MV, Buyanova ES, Petrova SA. Preparation, Structure and Characteristics of Solid Solutions La1-xBixMn1-yMyO3 (M = Fe, Ni, Cu). Chem Sustainable Development. 2016;24:135. doi:10.15372/KhUR20160203
Kaimieva OS, Danilova VV, Kruzhkov DA, Buyanova ES, Petrova SA. The solid Solutions Based on Lanthanum Manganite as the Cathod Materials for Bismuth-Containing Solid Electrolytes. Russ J Electrochem. 2017;53:852–8. doi:10.1134/S1023193517080080
Kaymieva OS, Morozova MV, Buyanova ES, Mikhailovskaya ZA, Petrova SA, Tarakina NV. Crystal Structure and Characterization of La1-xBixMnO3+δ. ECS Trans. 2015;68:977–85. doi:10.1149/06801.0977ecst
Wincewicz KC, Cooper JS. Taxonomies of SOFC material and manufacturing alternatives. J Power Sources. 2005;140:280–96. doi:10.1016/j.jpowsour.2004.08.032
West AR. Solid State Chemistry and Its Application. New York: John Wiley & Sons; 1987. 742 p.
DOI: https://doi.org/10.15826/chimtech.2018.5.3.03
Copyright (c) 2018 Olga Kaimieva, Valeria Danilova, Elena Buyanova, Sofia Petrova, Irina Nikolaenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






