Nucleophilic conjugate trifluoromethylation of chromones and activated alkenes under the action of Ruppert’s reagent
Abstract
Keywords
Full Text:
PDFReferences
Langlois BR, Billard T. Some Recent Results in Nucleophilic Trifluoromethylation and Introduction of Fluorinated Moieties. Synthesis. 2003;2:185–94. doi:10.1055/s-2003-36812
Prakash GKS, Yudin AK. Perfluoroalkylation with Organosilicon Reagents. Chem Rev. 1997;97(3):757–86. doi:10.1021/cr9408991
Singh RP, Shreeve JM. Nucleophilic Trifluoromethylation Reactions of Organic Compounds with (Trifluoromethyl)trimethylsilane. Tetrahedron. 2000;56(39):7613–32. doi:10.1016/S0040-4020(00)00550-0
Prakash GKS, Mandal M. Nucleophilic trifluoromethylation tamed. J Fluorine Chem. 2001;112(1):123–31. doi:10.1016/S0022-1139(01)00477-8
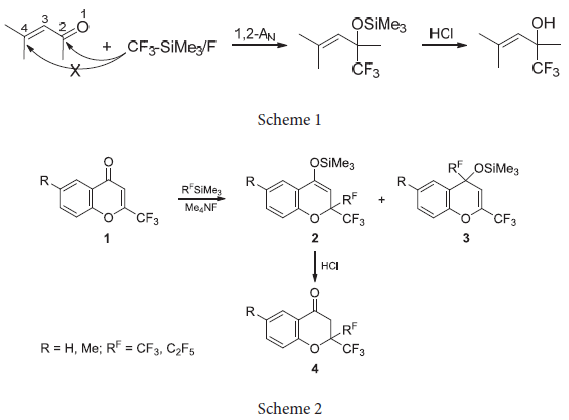
Sosnovskikh VYa, Sevenard DV, Usachev BI, Röschenthaler GV. The first example of a preparative 1,4-perfluoroalkylation using (perfluoroalkyl)trimethylsilanes. Tetrahedron Lett. 2003;44(10):2097–9. doi:10.1016/S0040-4039(03)00184-9
Sosnovskikh VYa, Usachev BI, Sevenard DV, Röschenthaler GV. Regioselective Nucleophilic 1,4-Trifluoromethylation of 2-Polyfluoroalkylchromones with (Trifluoromethyl)trimethylsilane. Synthesis of Fluorinated Analogs of Natural 2,2-Dimethylchroman-4-ones and 2,2-Dimethylchromenes. J Org Chem. 2003;68(20):7747–54. doi:10.1021/jo034591y
Ayer WA, Trifonov LS. Anthraquinones and a 10-Hydroxyanthrone from Phialophora alba. J Nat Prod. 1994;57(2):317-9. doi:10.1021/np50104a021
Kamat VP, Asolkar RN, Kirtany JK. A short and facile synthesis of lactarochromal and the corresponding acid. J Chem Research. 2001;1:41. doi:10.3184/030823401103168235
le-van Ngo, Van Cuong Pham Thi. An unusual m-hydroxyacetophenone and three new chromanone derivatives from Chrysothamnus viscidiflorus. Phytochemistry. 1981;20(3):485–7. doi:10.1016/S0031-9422(00)84171-0
Bowers WS, Ohta T, Cleere JS, Marsella PA. Discovery of insect anti-juvenile hormones in plants. Science. 1976;193(4253):542–7. doi:10.1126/science.986685
Sosnovskikh VYa, Usachev BI, Vorontsov II. 7-Polyfluoroalkylnorkhellins: synthesis and reactions with alkyl mercaptoacetates. Tetrahedron. 2003;59(14):2549–54. doi:10.1016/S0040-4020(03)00252-7
Sosnovskikh VYa, Usachev BI. 2-Polyfluoroalkylchromones. 13. Synthesis and nitration of 6,8-dibromo-2-trifluoromethylchromone. Russ Chem Bull. 2002;51(10):1954–56. doi:10.1023/A:1021385409506
Usachev BI, Sosnovskikh VYa. A simple and highly efficient synthesis of N-phenyl-2-polyfluoroalkyl-4-quinolones from 2-anilinoacetophenone and RFCO2Et. J Fluorine Chem. 2004;125(9):1393–5. doi:10.1016/j.jfluchem.2004.04.010
Sosnovskikh VYa, Barabanov MA. The first synthesis of 8-aza-2-polyfluoroalkylchromones. J Fluorine Chem. 2003;120(1):25–8. doi:10.1016/S0022-1139(02)00280-4
Sosnovskikh VYa, Usachev BI, Sevenard DV, Röschenthaler GV. Nucleophilic trifluoromethylation of RF-containing 4-quinolones, 8-aza- and 1-thiochromones with (trifluoromethyl)trimethylsilane. J Fluorine Chem. 2005;126(5):779–84. doi:10.1016/j.jfluchem.2005.03.001
Usachev BI, Sosnovskikh VYa, Shafeev MA, Röschenthaler G-V. A Novel and Simple Synthesis of 2-(Trifluoromethyl)-4H-thiochromen-4-ones. Phosphorus, Sulfur, and Silicon and the Related Elements. 2005;180(5-6):1315–9. doi:10.1080/10426500590912259
Sevenard DV, Sosnovskikh VYa, Kolomeitsev AA, Königsmann MH, Röschenthaler GV. Regioselective 1,4-trifluoromethylation of α,β-enones using ‘protect-in-situ’ methodology. Tetrahedron Lett. 2003;44(41):7623–7. doi:10.1016/j.tetlet.2003.08.050
Wang CL, Li HQ, Meng WD, Qing FL. Trifluoromethylation of flavonoids and anti-tumor activity of the trifluoromethylated flavonoid derivatives. Bioorg Med Chem Lett. 2005;15(20):4456–8. doi:10.1016/j.bmcl.2005.07.047
Sosnovskikh VYa, Usachev BI, Permyakov MN, Sevenard DV, Röschenthaler GV. First example of regioselective nucleophilic 1,6-addition of trimethyl(trifluoromethyl)silane to 4H-chromene derivatives. Russ Chem Bull. 2006;55(9):1687–89. doi:10.1007/s11172-006-0475-9
Dilman AD, Levin VV, Belyakov PA, Struchkova MI, Tartakovsky VA. Nucleophilic trifluoromethylation of arylidenemalononitriles. Tetrahedron Lett. 2008;49(28):4352–4. doi:10.1016/j.tetlet.2008.05.039
Zemtsov AA, Levin VV, Dilman AD, Struchkova MI, Belyakov PA, Tartakovsky VA. Nucleophilic trifluoromethylation of arylidene Meldrum’s acids. Tetrahedron Lett. 2009;50(25):2998–3000. doi:10.1016/j.tetlet.2009.03.188
Zemtsov AA, Levin VV, Dilman AD, Struchkova MI, Tartakovsky VA. Reactions of fluorinated silanes with 2-nitrocinnamates. J Fluorine Chem. 2011;132(6):378–81. doi:10.1016/j.jfluchem.2011.03.015
DOI: https://doi.org/10.15826/chimtech.2017.4.1.022
Copyright (c) 2017 V. Ya. Sosnovskikh, G.-V. Roshentaler

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






