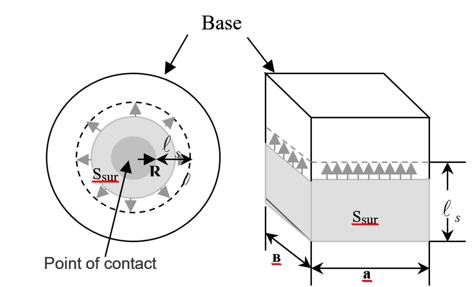
Surface reactions with participation of oxides of molybdenum and tungsten
Abstract
Keywords
Full Text:
PDFReferences
Neiman AYa, Guseva AF. New Data on the Mechanism of Mass Transfer in Solid-Phase Reactions: II. Chemical and Electrochemical Surface Phenomena. Kinet Catal. 1999;40(I):38-49.
Neiman AYa, Guseva AF, Trifonova MV. Surface Reaction in the course of molybdates and tungstates formation. Solid State Ionics. 2001;141-2:321-9. doi:10.1016/S0167-2738(01)00798-6
Neiman AYa, Guseva AF, Trifonova MV, Sukhankina IV. Reactive Surface Diffusion during Synthesis of Molybdates and Tungstates: The Role of Phase Constitution of Product. Russ J Inorg Chem. 2005;50:319-24.
Chebotin VN, Perfil'ev MV. Electrokhimiya tverdykh elektrolitov [Electrochemistry of Solid Electrolytes]. Moscow: Khimiya; 1978. 312 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2015.2.4.033
Copyright (c) 2015 A. F. Guseva, M. V. Trifonova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






