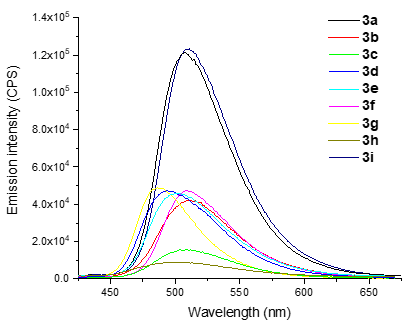
4,5-Diaryl-3-hydroxy-2,2’-bipyridine-6-carbonitriles as prospective luminophores
Abstract
Keywords
Full Text:
PDFReferences
Kaczmarek U, Balicki R, Lipkowski J, Borowicz P, Grabowska A. Structure and Photophysics of Deazabipyridyls. Excited Internally Hydrogen-Bonded Systems with One Proton Transfer Reaction Site. J Chem Soc Perkin Trans. 1994;7:1603–1610. doi:10.1039/p29940001603
Borowicz P, Grabowska A, Leś A, Kaczmarek Ł, Zagrodzki B. New Phototautomerizing Systems: Non-Symmetric Derivatives of [2,2′-Bipyridyl]-3,3′-Diol. Chem Phys Lett. 1998;291(3–4):351–359. doi:10.1016/S0009-2614(98)00565-X
Ulrich G, Nastasi F, Retailleau P, Puntoriero F, Ziessel R, Campagna S. Luminescent Excited‐State Intramolecular Proton‐Transfer (ESIPT) Dyes Based on 4‐Alkyne‐Functionalized [2,2′‐Bipyridine]‐3,3′‐diol Dyes. Chem A Eur J 2008;14(14):4381–4392. doi:10.1002/chem.200701803
Trannoy V, Léaustic A, Gadan S, Guillot R, Allain C, Clavier G, Mazerat S, Geffroy B, Yu PA. Highly Efficient Solution and Solid State ESIPT Fluorophore and Its OLED Application. New J Chem. 2021;45(6):3014–3021. doi:10.1039/D0NJ05600F
Sepioł J, Bulska H, Grabowska A. The Dihydroxy Derivative of 2,2’-Bipyridyl as a New Proton-Transfer Lasing Dye. Chem Phys Lett. 1987:140(6):607–610. doi:10.1016/0009-2614(87)80496-7
Rurack K, Hoffmann K, Al-Soufi W, Resch-Genger U. 2,2‘-Bipyridyl-3,3‘-Diol Incorporated into AlPO4 -5 Crystals and Its Spectroscopic Properties as Related to Aqueous Liquid Media. J Phys Chem B. 2002;106(38):9744–9752. doi:10.1021/jp0259622
Reynal A, Etxebarria J, Nieto N, Serres S, Palomares E, Vidal‐Ferran A. A Bipyridine‐Based “Naked‐Eye” Fluorimetric Cu2+ Chemosensor. Eur J Inorg Chem. 2010;2010(9):1360–1365. doi:10.1002/ejic.200900887
Mandal S, Ghosh S, Banerjee C, Kuchlyan J, Sarkar N. Unique Photophysical Behavior of 2,2′-Bipyridine-3,3′-Diol in DMSO–Water Binary Mixtures: Potential Application for Fluorescence Sensing of Zn2+ Based on the Inhibition of Excited-State Intramolecular Double Proton Transfer. J Phys Chem B. 2013;117(40):12212–12223. doi:10.1021/jp406853r
Mandal S, Ghosh S, Aggala HHK, Banerjee C, Rao VG, Sarkar N. Modulation of the Photophysical Properties of 2,2′-Bipyridine-3,3′-Diol inside Bile Salt Aggregates: A Fluorescence-Based Study for the Molecular Recognition of Bile Salts. Langmuir. 2013;29(1):133–143. doi:10.1021/la304319r
Zhao X, Zhu L, Li Q, Yin H, Shi Y. The Interplay between ESIPT and TADF for the 2,2′-Bipyridine-3,3′-Diol: A Theoretical Reconsideration. IJMS. 2022;23(22):13969. doi:10.3390/ijms232213969
Huang J, Bai X, Li L, Zheng Z, Xu Z, Cui Y, Cao J, Xu L. Transition‐Metal‐Free Oxidative C(Sp2)−H Hydroxylation of Terpyridines: A HOMO‐Raising Strategy for the Construction of a New Super‐Stable Terpyridine Chromophores. Chem A Eur J. 2017;23(17):4055–4059. doi:10.1002/chem.201605793
Rammohan A, Krinochkin AP, Kopchuk DS, Shtaitz YK, Savchuk MI, Starnovskaya ES, Zyryanov GV, Rusinov VL, Chupakhin ON. Synthesis and Photoluminescent Properties of 4,5-Diaryl-3-Hydroxy- and 3-Methoxypyridine-6-Carbonitriles. Russ J Org Chem. 2022;58(2):180–183. doi:10.1134/S1070428022020038
Mongin F, Trécourt F, Mongin O, Quéguiner G. New Syntheses of Orelline and Analogues via Metalation and Cross-Coupling Reactions. Tetrahedron. 2002;58(2):309–314. doi:10.1016/S0040-4020(01)01142-5
Huo W, Miki K, Tokunaga D, Mu H, Oe M, Harada H, Ohe K. Dual-Stimuli-Responsive Probes for Detection of Ovarian Cancer Cells and Quantification of Both pH and Enzyme Activity. Bull Chem Soc Japan. 2021;94(8):2068–2075. doi:10.1246/bcsj.20210168
Forshaw S, Knighton RC, Reber J, Parker JS, Chmel NP, Wills M. A Strained Alkyne-Containing Bipyridine Reagent; Synthesis, Reactivity and Fluorescence Properties. RSC Adv. 2019;9(62):36154–36161. doi:10.1039/C9RA06866J
Yang PS, Tsai MT, Tsai MH, Ong CW. The Regioselective Homocoupling of Meta‐Hydroxypyridines with Hypervalent Iodine(III). Chem Asian J. 2015;10(4):849–852. doi:10.1002/asia.201403359
Zhang S, Zhang D, Liebeskind LS. Ambient Temperature, Ullmann-like Reductive Coupling of Aryl, Heteroaryl, and Alkenyl Halides. J Org Chem. 1997;62(8):2312–2313. doi:10.1021/jo9700078
Chubb RWJ, Bryce MR, Tarbit B. Synthesis of 2-Heteroaryl-3-Hydroxypyridines by Ring Expansion Reactions of 2-Acylfurans with Ammonia. J Chem Soc Perkin Trans 1. 2001;16:1853–1854. doi:10.1039/b105228b
Gao L, Delle Piane M, Corno M, Jiang F, Raja R, Pera-Titus M. Synthesis of Amine Derivatives from Furoin and Furil over a Ru/Al2O3 Catalyst. Catal Sci Technol. 2024;14(9):2593–2599. doi:10.1039/D3CY01605F
Kozhevnikov VN, Kozhevnikov DN, Nikitina TV, Rusinov VL, Chupakhin ON, Zabel M, König B. A Versatile Strategy for the Synthesis of Functionalized 2,2‘-Bi- and 2,2‘:6‘,2‘ ‘-Terpyridines via Their 1,2,4-Triazine Analogues. J Org Chem. 2003;68(7):2882–2888. doi:10.1021/jo0267955
Krinochkin AP, Mallikarjuna RG, Kopchuk DS, Slepukhin PA, Shtaitz YK, Khalymbadzha IA, Kovalev IS, Kim GA, Ganebnykh IN, Zyryanov GV, Chupakhin ON, Charushin VN. 2-Aminooxazoles as Novel Dienophiles in the Inverse Demand Diels–Alder Reaction with 1,2,4-Triazines. Mendeleev Comm. 2021;31(4):542–544. doi:10.1016/j.mencom.2021.07.035
Rammohan A, Krinochkin AP, Kopchuk DS, Shtaitz YaK, Kovalev IS, Savchuk MI, Zyryanov GV, Rusinov VL, Chupakhin ON. Conditions for the Synthesis of 4,5-Diaryl-3-Hydroxy-2,2’-Bipyridine-6-Carbonitriles by the Reaction of 1,2,4-Triazine-5-Carbonitriles with 2-Aminooxazoles. Russ J Org Chem. 2022;58(2):175–179. doi:10.1134/S1070428022020026
Krinochkin AP, Valieva MI, Starnovskaya ES, Shtaitz YaK, Rybakova SS, Sharafieva ER, Kopchuk DS, Zyryanov GV, Rusinov VL. The Synthetic Approaches to (3-Thienyl)-Containing 2,2’-Bipyridines as Potential Monomers for Electropolymerization. Dokl Chem. 2022;506(1):196–201. doi:10.1134/S0012500822700100
Pattanayak BK, Rout DN, Mahapatra GN. Studies on Oxazoles. Part I : Synthesis and Chlorination of Some New 2-Amino-4-Substituted Oxazoles and Their Use as Fungicides. J Indian Chem Soc. 1978;55:264–267. doi:10.5281/ZENODO.6379265
Slepukhin PA, Rybakova AV, Gaviko VS, Rammohan A, Shtaitz YaK, Krinochkin AP, Ladin ED, Kopchuk DS, Zyryanov GV, Rusinov VL. Single-Crystal X-Ray Diffraction Study of 4,5-Diaryl-3-Hydroxy-2,2′-Bipyridine-6-Carbonitriles. Russ Chem Bull. 2023;72(12):2825–2836. doi:10.1007/s11172-023-4091-8
Porrès L, Holland A, Pålsso, L-O, Monkman AP, Kemp C, Beeby A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J Fluoresc. 2006;16(2):267–273. doi:10.1007/s10895-005-0054-8
Sedgwick AC, Wu L, Han H-H, Bull SD, He X-P, James TD, Sessler JL, Tang BZ, Tian H, Yoon J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem Soc Rev. 2018;47(23):8842–8880. doi:10.1039/C8CS00185E
Liu X, Xu Z, Cole JM. Molecular Design of UV–Vis Absorption and Emission Properties in Organic Fluorophores: Toward Larger Bathochromic Shifts, Enhanced Molar Extinction Coefficients, and Greater Stokes Shifts. J Phys Chem C 2013;117(32):16584–16595. doi:10.1021/jp404170w
Rodrigues ACB, Seixas De Melo JS. Aggregation-Induced Emission: From Small Molecules to Polymers—Historical Background, Mechanisms and Photophysics. Top Curr Chem (Z). 2021;379(3):15. doi:10.1007/s41061-021-00327-9
Dong L, Peng HQ, Niu LY, Yang QZ. Modulation of Aggregation-Induced Emission by Excitation Energy Transfer: Design and Application. Top Curr Chem (Z). 2021;379(3):18. doi:10.1007/s41061-021-00330-0
Gao M, Tang BZ. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017;2(10):1382–1399. doi:10.1021/acssensors.7b00551
Lakowicz JR (2006) Principles of Fluorescence Spectroscopy. Springer, Springer US, Boston, MA. doi:10.1007/978-0-387-46312-4
Zhang SG, Hou C. Aquabis(3′-Hydroxy-2,2′-Bipyridine-3-Olato-κ2 N , N ′)Zinc(II). Acta Crystallogr E Struct Rep Online. 2008;64(9):m1131–m1131. doi:10.1107/S1600536808024495
Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the Physiology and Pathology of the CNS. Nat Rev Neurosci. 2009;10(11):780–791. doi:10.1038/nrn2734
Berg JM, Shi Y. The Galvanization of Biology: A Growing Appreciation for the Roles of Zinc. Sci. 1996;271(5252):1081–1085. doi:10.1126/science.271.5252.1081
DOI: https://doi.org/10.15826/chimtech.2025.12.2.10
Copyright (c) 2025 Evgeny D. Ladin, Yaroslav K. Shtaitz, Vladimir V. Chernyshov, Ilia A. Eliseev, Maria I. Valieva, Yulia M. Sayfutdinova, Svetlana E. Vatolina, Ksenia D. Krasnoperova, Youcef Ballou, Dmitry S. Kopchuk, Grigory V Zyryanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







