Structural and transport properties of La tungstate and its composite with nickel (II) and copper (II) oxides
Abstract
Keywords
Full Text:
PDFReferences
Liang W, Zhang Y, Hu T, Jiang H. Enhanced H2 production by using La5.5WO11.25-δ-La0.8Sr0.2FeO3-δ mixed oxygen ion-proton-electron triple-conducting membrane. Int J Hydrog Energy. 2021;46(66):33143–51. doi:10.1016/j.ijhydene.2021.07.134
Cao F, Ji X, Shao Z. Nanotechnologies in ceramic electrochemical cells. Chem Soc Rev. 2024;53(1):450–501. doi:10.1039/D3CS00303E
Malavasi L, Karlsson M, Coduri M. Structure-property correlation in oxide-ion and proton conductors for clean energy applications: Recent experimental and computational advancements. J Mater Chem A. 2022;10(10):5082–110. doi:10.1039/D1TA10326A
Osinkin D, Tropin E. Hydrogen production from methane and carbon dioxide mixture using all-solid-state electrochemical cell based on a proton-conducting membrane and redox-robust composite electrodes. J Energy Chem. 2022;69:576–84. doi:10.1016/j.jechem.2022.02.019
Osinkin DA. Electrochemical behaviour of redox-robust electrode in contact with protonic electrolyte: Case of double-layered Sr2Fe1.5Mo0.5O6-δ - Ce0.8Sm0.2O2-δ composite. Int J Hydrog Energy. 2024;77:1066–73. doi:10.1016/j.ijhydene.2024.06.266
Singh M, Paydar S, Singh AK, Singhal R, Singh A, Singh M. Recent advancement of solid oxide fuel cells towards semiconductor membrane fuel cells. Energy Mater. 2024;4:400012. doi:10.20517/energymater.2023.54
Plekhanov MS, Thomä SLJ, Zobel M, Cuello GJ, Fischer HE, Raskovalov AA, Kuzmin AV. Correlating proton diffusion in perovskite triple-conducting oxides with local and defect structure. Chem Mater. 2022;34(10):4785–94. doi:10.1021/acs.chemmater.2c01159
Saini N, Awasthi K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep Purif Technol. 2022;282B:120029. doi:10.1016/j.seppur.2021.120029
Alimov VN, Busnyuk AO, Kuzenov SR, Peredistov EU, Livshits AI. Bcc V–Fe alloys for the hydrogen separation membranes: Hydrogen solubility and global character of alloying effect. J Memb Sci. 2022;644:120159. doi:10.1016/j.memsci.2021.120159
Singla S, Shetti NP, Basu S, Mondal K, Aminabhavi TM. Hydrogen production technologies – Membrane based separation, storage and challenges. J Environ Manage. 2022;302A:113963. doi:10.1016/j.jenvman.2021.113963
Bernardo G, Araújo T, da Silva Lopes T, Sousa J, Mendes A. Recent advances in membrane technologies for hydrogen purification. Int J Hydrog Energy. 2020;45(12):7313–38. doi:10.1016/j.ijhydene.2019.06.162
Zhang Q, Liu T, Zhu Z, Hao L, Liu W. Modeling of hydrogen permeation for Ni–ceramic proton conductor composite membrane with symmetric structure. J Memb Sci. 2012;415–16:328–35. doi:10.1016/j.memsci.2012.05.023
Poetzsch D, Merkle R, Maier J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys Chem Chem Phys. 2014;16(31):16446–53. doi:10.1039/C4CP00459K
Escolástico S, Somacescu S, Serra JM. Tailoring mixed ionic–electronic conduction in H2 permeable membranes based on the system Nd5.5W1−xMoxO11.25−δ. J Mater Chem A. 2015;3(2):719–31. doi:10.1039/C4TA03699A
Cheng H. Rare earth tungstate: One competitive proton conducting material used for hydrogen separation: A review. Separations. 2023;10(5):317. doi:10.3390/separations10050317
Fontaine M, Norby T, Larring Y, Grande T, Bredesen R. Oxygen and hydrogen separation membranes based on dense ceramic conductors. In: Membrane Science and Technology, Volume 13. Elsevier; 2008. pp. 401–58. doi:10.1016/S0927-5193(07)13010-2
Bespalko Y, Sadykov V, Eremeev N, Skryabin P, Krieger T, Sadovskaya E, Bobrova L, Uvarov N, Lukashevich A, Krasnov A, Fedorova Y. Synthesis of tungstates/Ni0.5Cu0.5O nanocomposite materials for hydrogen separation cermet membranes. Compos Struct. 2018;202:1263–74. doi:10.1016/j.compstruct.2018.06.004
Su H, Hu YH. Degradation issues and stabilization strategies of protonic ceramic electrolysis cells for steam electrolysis. Energy Sci Eng. 2022;10(5):1706–25. doi:10.1002/ese3.1010
Shirbhate S, Acharya S. Polyvinyl alcohol/polybenzimidazole/BaZrO3–based hybrid nanocomposite: As a new proton conducting membrane for proton exchange membrane fuel cells. Ferroelectrics. 2022;587(1):118–26. doi:10.1080/00150193.2022.2034421
Long Y, Yang K, Gu Z, Lin S, Li D, Zhu X, Wang H, Li K. Hydrogen generation from water splitting over polyfunctional perovskite oxygen carriers by using coke oven gas as reducing agent. Appl Catal B Environ. 2022;301:120778. doi:10.1016/j.apcatb.2021.120778
Escolástico S, Solís C, Scherb T, Schumacher G, Serra JM. Hydrogen separation in La5.5WO11.25−δ membranes. J Memb Sci. 2013;444:276–84. doi:10.1016/j.memsci.2013.05.005
Hancke R, Sarah F, Kilner JA, Haugsrud R. Determination of proton- and oxide ion tracer diffusion in lanthanum tungstate (La/W= 5.6) by means of ToF-SIMS. Phys Chem Chem Phys. 2012;14(40):13971–8. doi:10.1039/C2CP42278F
Zayas-Rey MJ, dos Santos-Gómez L, Marrero-López D, León-Reina L, Canales-Vázquez J, Aranda MAG, Losilla ER. Structural and conducting features of niobium-doped lanthanum tungstate, La27(W1–xNbx)5O55.55−δ. Chem Mater. 2013;25(3):448–56. doi:10.1021/cm304067d
Escolástico S, Vert VB, Serra JM. Preparation and characterization of nanocrystalline mixed proton-electronic conducting materials based on the system Ln6WO12. Chem Mater. 2009;21(14):3079–89. doi:10.1021/cm900067k
Baidya A, Dutta A. Exploring phase transition and charge carrier dynamics in La6MoO12 ionic conductors: Impact of metal-substitution. Mater Res Bull. 2024;179:112968. doi:10.1016/j.materresbull.2024.112968
Escolástico S, Solís C, Serra JM. Study of hydrogen permeation in (La5/6Nd1/6)5.5WO12-δ membranes. Solid State Ionics. 2012;216:31–5. doi:10.1016/j.ssi.2011.11.004
Shlyakhtina A, Lyskov N, Baldin E, Stolbov D, Kolbanev I, Shatov A, Kasyanova A, Medvedev D. Impact of Ln cation on the oxygen ion conductivity of Ln14W4O33 (Ln= Nd, Sm, Gd, Dy, Ho, Er, Tm, Yb) tungstates. Ceram Int. 2024;50(1A):704–13. doi:10.1016/j.ceramint.2023.10.149
Magrasó A, Haugsrud R. Effects of the La/W ratio and doping on the structure, defect structure, stability and functional properties of proton-conducting lanthanum tungstate La28−xW4+xO54+δ. A review. J Mater Chem A. 2014;2(32):12630–41. doi:10.1039/C4TA00546E
Hancke R, Magrasó A, Norby T, Haugsrud R. Hydration of lanthanum tungstate (La/W=5.6 and 5.3) studied by TG and simultaneous TG–DSC. Solid State Ionics. 2013;231:25–9. doi:10.1016/j.ssi.2012.10.022
Bespalko Y, Eremeev N, Skryabin P, Krieger T, Chesalov Y, Lapina O, Khabibulin D, Ulihin A, Uvarov N, Sadykov V. Structural and transport properties of neodymium tungstates prepared via mechanochemical activation. Ceram Int. 2019;45(7B):9529–36. doi:10.1016/j.ceramint.2018.09.277
Sadykov V, Shlyakhtina A, Sadovskaya E, Eremeev N, Skazka V, Goncharov V. 2D diffusion of oxygen in Ln10Mo2O21 (Ln = Nd, Ho) oxides. Solid State Ionics. 2020;346:115229. doi:10.1016/j.ssi.2020.115229
Shlyakhtina AV, Lyskov NV, Šalkus T, Kežionis A, Patrakeev MV, Leonidov IA, Shcherbakova LG, Chernyak SA, Shefer KI, Sadovskaya EM, Eremeev NF, Sadykov VA. Conductivity and oxygen diffusion in bixbyites and fluorites Ln6−xMoO12−δ (Ln = Er, Tm; x = 0, 0.5). Int J Hydrog Energy. 2021;46(32):16965–76. doi:10.1016/j.ijhydene.2021.02.029
Gilev AR, Kiselev EA, Sukhanov KS, Korona DV, Cherepanov VA. Evaluation of La2-x(Ca/Sr)xNi1-yFeyO4+δ (x= 0.5, 0.6; y= 0.4, 0.5) as cathodes for proton-conducting SOFC based on lanthanum tungstate. Electrochim Acta. 2022;421:140479. doi:10.1016/j.electacta.2022.140479
Erdal S, Kalland L-E, Hancke R, Polfus J, Haugsrud R, Norby T, Magrasó A. Defect structure and its nomenclature for mixed conducting lanthanum tungstates La28–xW4+xO54+3x/2. Int J Hydrog Energy. 2012;37(9):8051–5. doi:10.1016/j.ijhydene.2011.11.093
Magrasó A. Transport number measurements and fuel cell testing of undoped and Mo-substituted lanthanum tungstate. J Power Sources. 2013;240:583–8. doi:10.1016/j.jpowsour.2013.04.087
Magrasó A, Frontera C, Marrero-López D, Núñez P. New crystal structure and characterization of lanthanum tungstate “La6WO12” prepared by freeze-drying synthesis. Dalton Trans. 2009;46:10273–83. doi:10.1039/B916981B
Jayalekshmy NL, John A, Thomas JK, Solomon S. Structural, optical and electrical characterizations of Ln6WO12 (Ln = La, Nd, Sm, Gd) nanoceramics. Appl Phys A. 2019;125(2):143. doi:10.1007/s00339-019-2441-z
Polfus JM, Xing W, Fontaine ML, Denonville C, Henriksen PP, Bredesen R. Hydrogen separation membranes based on dense ceramic composites in the La27W5O55.5–LaCrO3 system. J Memb Sci. 2015;479:39–45. doi:10.1016/j.memsci.2015.01.027
Partin GS, Korona DV, Neiman AYa, Belova KG. Conductivity and hydration of fluorite-type La6−xWO12−1.5x phases (x= 0.4; 0.6; 0.8; 1). Russ J Electrochem. 2015;51(5):381–90. doi:10.1134/S1023193515050092
Cao Y, Duan N, Jian L, Evans A, Haugsrud R. Effect of Nb doping on hydration and conductivity of La27W5O55.5−δ. J Am Ceram Soc. 2016;99(10):3309–16. doi:10.1111/jace.14346
Hancke R, Li Z, Haugsrud R. Thermogravimetric relaxation study of the proton conductor lanthanum tungstate, La28−xW4+xO54+δv2−δ, x = 0.85. Int J Hydrog Energy. 2012;37(9):8043–50. doi:10.1016/j.ijhydene.2011.11.050
Surzhikov AP, Malyshev AV, Lysenko EN, Stary O. Temperature dependences of the initial permeability of lithium-titanium ferrites produced by solid-state sintering in thermal and radiation-thermal modes. Eurasian Phys Tech J. 2022;19(1(39)):5–9. doi:10.31489/2022No1/5-9
Lyakhov NZ, Boldyrev VV, Voronin AP, Gribkov OS, Bochkarev LG, Rusakov SV, Auslender VL. Electron beam stimulated chemical reaction in solids. J Therm Anal. 1995;43(1):21–31. doi:10.1007/bf02635965
Auslender VL, Bochkarev IG, Boldyrev VV, Lyakhov NZ, Voronin AP. Electron beam induced diffusion controlled reaction in solids. Solid State Ionics. 1997;101–3(1):489–93. doi:10.1016/S0167-2738(97)84073-8
Eremeev NF, Bespalko YuN, Sadovskaya EM, Skriabin PI, Krieger TA, Ishchenko AV, Sadykov VA. Structural and transport properties of Nd tungstates and their composites with Ni0.5Cu0.5O obtained by mechanical activation. Dalton Trans. 2022;51(19):7705–14. doi:10.1039/d2dt00498d
Kostishyn VG, Komlev AS, Korobeynikov MV, Bryazgin AA, Shvedunov VI, Timofeev AV, Mikhailenko MA. Effect of a temperature mode of radiation-thermal sintering the structure and magnetic properties of Mn-Zn-ferrites. J Nano-Electron Phys. 2015;7(4):04044.
TOPAS-Academic [Internet]. 2020[Cited 2024]. Available from: http://www.topas-academic.net/, Accessed on 11 December 2024
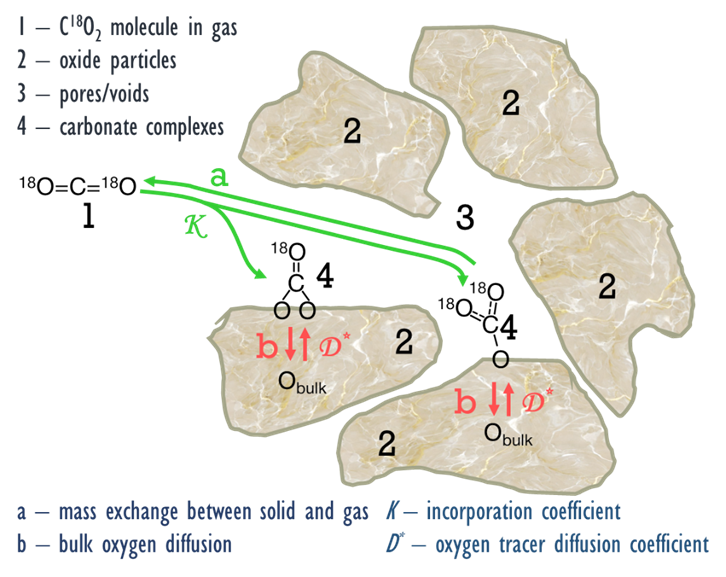
Sadykov V, Sadovskaya E, Bobin A, Kharlamova T, Uvarov N, Ulikhin A, Argirusis C, Sourkouni G, Stathopoulos V. Temperature-programmed C18O2 SSITKA for powders of fast oxide-ion conductors: estimation of oxygen self-diffusion coefficients. Solid State Ionics. 2015;271:69–72. doi:10.1016/j.ssi.2014.11.004
Sadykov VA, Sadovskaya EM, Uvarov NF. Methods of isotopic relaxations for estimation of oxygen diffusion coefficients in solid electrolytes and materials with mixed ionic-electronic conductivity. Russ J Electrochem. 2015;51(5):458–67. doi:10.1134/S1023193515050109
Sadykov VA, Sadovskaya EM, Eremeev NF, Skriabin PI, Krasnov AV, Bespalko YuN, Pavlova SN, Fedorova YuE, Pikalova EYu, Shlyakhtina AV. Oxygen mobility in the materials for solid oxide fuel cells and catalytic membranes (Review). Russ J Electrochem. 2019;55(8):701–18. doi:10.1134/S1023193519080147
Muzykantov VS, Popovski VV, Boreskov GK. Kinetika izotopnogo obmena v sisteme molekulyarnyi kislorod - tvyodyi okisel [Kinetics of isotope exchange in molecular oxygen - solid oxide system]. Kinet Katal. 1964;5(4):624–9. Russian.
Seeger J, Ivanova ME, Meulenberg WA, Sebold D, Stöver D, Scherb T, Schumacher G, Escolástico S, Solís C, Serra JM. Synthesis and characterization of nonsubstituted and substituted proton-conducting La6–xWO12–y. Inorg Chem. 2013;52(18):10375–86. doi:10.1021/ic401104m
Atas MS. The relationship between reinforcement ratio and e-beam irradiation in Y2O3 reinforced Al6061 alloys: A crystallographic assessment. Nucl Instrum Methods Phys Res B. 2024;548:165252. doi:10.1016/j.nimb.2024.165252
Pavlov YuS, Petrenko VV, Alekseev PA, Bystrov PA, Souvorova OA. Trends and opportunities for the development of electron-beam energy-intensive technologies. Radiat Phys Chem. 2022;198:110199. doi:10.1016/j.radphyschem.2022.110199
Pikaev A.K. Sovremennaya Radiatsionnaya Khimiya. Osnovnyye Polozheniya, Experimental’naya Tekhnika i Metody [Modern Radiation Chemistr. Basics, Experimental Equipment and Methods]. Moscow: Nauka; 1985. 374 p. Russian.
Stepanov VA. Radiation-stimulated diffusion in solids. Tech Phys. 1998;43(8):938–42. doi:10.1134/1.1259104
Bespalko Yu, Eremeev N, Sadovskaya E, Krieger T, Bulavchenko O, Suprun E, Mikhailenko M, Korobeynikov M, Sadykov V. Synthesis and oxygen mobility of bismuth cerates and titanates with pyrochlore structure. Membranes. 2023;13(6):598. doi:10.3390/membranes13060598
Sadykov V, Bespalko Yu, Sadovskaya E, Krieger T, Belyaev V, Eremeev N, Mikhailenko M, Bryazgin A, Korobeynikov M, Ulihin A, Uvarov N. Structural and transport properties of e-beam sintered lanthanide tungstates and tungstates-molybdates. Nanomater. 2022;12(19):3282. doi:10.3390/nano12193282
Sadykov VA, Shlyakhtina AV, Lyskov NV, Sadovskaya EM, Cherepanova SV, Eremeev NF, Skazka VV, Goncharov VB, Kharitonova EP. Oxygen diffusion in Mg-doped Sm and Gd zirconates with pyrochlore structure. Ionics. 2020;26(9):4621–33. doi:10.1007/s11581-020-03614-5
Sadykov VA, Bespalko YuN, Pavlova SN, Skriabin PI, Krasnov AV, Eremeev NF, Krieger TA, Sadovskaya EM, Belyaev VD, Vinokurov ZS. Protonic mobility of neodymium tungstate. J Electrochem En Conv Stor. 2017;14(4):044501. doi:10.1115/1.4037957
Porotnikova NM, Ananyev MV. Applicability of gas-phase isotope exchange method for investigation of porous materials. J Solid State Electrochem. 2021;25(4):1151–9. doi:10.1007/s10008-020-04896-5
Ren R, Sun J, Wang G, Xu C, Qiao J, Sun W, Wang Z, Sun K. Rational design of Sr2Fe1.5Mo0.4Y0.1O6-δ oxygen electrode with triple conduction for hydrogen production in protonic ceramic electrolysis cell. Sep Purif Technol. 2022;299:121780. doi:10.1016/j.seppur.2022.121780
Kreuer KD. Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ionics. 1999;125 (1–4):285–302. doi:10.1016/S0167-2738(99)00188-5
Farlenkov AS, Vlasov MI, Porotnikova NM, Bobrikov IA, Khodimchuk AV, Ananyev MV. Hydrogen diffusivity in the Sr-doped LaScO3 proton-conducting oxides. Int J Hydrog Energy. 2020;45(43):23455–6810. doi:10.1016/j.ijhydene.2020.06.148
Edwards AG. Measurement of the diffusion rate of hydrogen in nickel. Br J Appl Phys. 1958;8(10):406–10. doi:10.1088/0508-3443/8/10/306
Piper J. Diffusion of hydrogen in copper‐palladium alloys. J Appl Phys. 1966;37(2):715–21. doi:10.1063/1.1708243
Huang F, Li X, Shan X, Guo J, Gallucci F. Annaland MVS, Liu D. Hydrogen transport through the V-Cr-Al Alloys: Hydrogen solution, permeation and thermal-stability. Sep Purif Technol 2020;240:116654. doi:10.1016/j.seppur.2020.116654
Sadovskaya EM, Bobin AS, Skazka VV. Isotopic transient analysis of oxygen exchange over oxides. Chem Eng J. 2018;348:1025–36. doi:10.1016/j.cej.2018.05.027
Ananyev MV, Kurumchin EKh, Porotnikova NM. Effect of oxygen nonstoichiometry on kinetics of oxygen exchange and diffusion in lanthanum-strontium cobaltites. Russ J Electrochem. 2010;46(7):789–97. doi:10.1134/S1023193510070128
Sadykov V, Eremeev N, Sadovskaya E, Zhulanova T, Pikalov S, Fedorova Yu, Pikalova E. Impact of calcium and copper co-doping on the oxygen transport of layered nickelates: A case study of Pr1.6Ca0.4Ni1–yCuyO4+δ and a comparative analysis. Chim Tech Acta. 2024;11(4): 202411411. doi:10.15826/chimtech.2024.11.4.11
Geffroy P-M, Deronzier E, Gillibert J, Munch P, Chartier T, Fouletier J. Determination of oxygen diffusion and surface exchange coefficients of mixed ionic-electronic conductors by oxygen semi-permeation methods. J Electrochem Soc. 2020;167(6):064503. doi:10.1149/1945-7111/ab7b84
Wachsman ED, Boyapati S, Kaufman MJ, Jiang N. Modeling of ordered structures of phase-stabilized cubic bismuth oxides. J Am Ceram Soc. 2000;83(8):1964–8. doi:10.1111/j.1151-2916.2000.tb01498.x
Li Q, Thangadurai V. Synthesis, structure and electrical properties of Mo-doped CeO2–materials for SOFCs. Fuel Cells. 2009;9(5):684–98. doi:10.1002/fuce.200900044
Kalland L-E, Magrasó A, Mancini A, Tealdi C, Malavasi L. Local structure of proton-conducting lanthanum tungstate La28–xW4+xO54+δ: A combined density functional theory and pair distribution function study. Chem Mater. 2013;25(11):2378–84. doi:10.1021/cm401466r
Kalland L-EQ. Ab initio Modelling and Experimental Studies of Order-Disorder, Hydration, and Ionic Conductivity of Fluorite Related Oxides [PhD Dissertation]. Oslo (Kingdom of Norway): University of Oslo; 2021. 123 p.
Baldin E, Lyskov N, Vorobieva G, Kolbanev I, Karyagina O, Stolbov D, Voronkova V, Shlyakhtina A. Synthesis of hexagonal nanophases in the La2O3–MO3 (M = Mo, W) systems. Energies. 2023;16(15):5637. doi:10.3390/en16155637
Zhu Y, Wang J, Rykov AI, Zhu X, Yang W. Oxygen transport kinetics affected by grain size – A permeation model study. J Memb Sci. 2020;603:118038. doi:10.1016/j.memsci.2020.118038
Chen Y, Wei Y, Xie H, Zhuang L, Wang H. Effect of the La/W ratio in lanthanum tungstate on the structure, stability and hydrogen permeation properties. J Memb Sci. 2017;542:300–6. doi:10.1016/j.memsci.2017.08.031
Amsif M, Magrasó A, Marrero-López D, Ruiz-Morales JC, Canales-Vázquez J, Núñez, P. Mo-substituted lanthanum tungstate La28–yW4+yO54+δ: A competitive mixed electron–proton conductor for gas separation membrane applications. Chem Mater. 2012;24(20):3868–77. doi:10.1021/cm301723a
Vøllestad E, Gorzkowska-Sobas A, Haugsrud R. Fabrication, structural and electrical characterization of lanthanum tungstate films by pulsed laser deposition. Thin Solid Films. 2012;520(21):6531‒4. doi:10.1016/j.tsf.2012.06.060
Kojo G, Shono Y, Ushiyama H, Oshima Y, Otomo J. Influence of La/W ratio on electrical conductivity of lanthanum tungstate with high La/W ratio. J Solid State Chem. 2017;248:1–8. doi:10.1016/j.jssc.2017.01.011
Escolástico S, Balaguer M, Solís C, Toldra-Reig F, Somacescu S, Gerhards U, Aguadero A, Haas-Santo K, Dittmeyer R, Serra JM. Promotion of mixed protonic–electronic transport in La5.4WO11.1−δ membranes under H2S atmospheres. J Mater Chem A. 2023;11(32):17246–56. doi:10.1039/d3ta01827j
De Souza RA, Pietrowski MJ, Anselmi-Tamburini U, Kim S, Munirb ZA, Martin M. Oxygen diffusion in nanocrystalline yttria-stabilized zirconia: The effect of grain boundaries. Phys Chem Chem Phys. 2008;10(15):2067–72. doi:10.1039/B719363G
Jiang N. Electron irradiation effects in transmission electron microscopy: Random displacements and collective migrations. Micron. 2023;171:103482. doi:10.1016/j.micron.2023.103482
Sadykov VA, Sadovskaya EM, Bespalko YuN, Smal’ EA, Eremeev NF, Prosvirin IP, Bulavchenko OA, Mikhailenko MA, Korobeynikov MV. Structural, surface and oxygen transport properties of Sm-doped Nd nickelates. Solid State Ionics. 2024;412:116596. doi:10.1016/j.ssi.2024.116596
Sadykov VA, Bespalko YuN, Krasnov AV, Skriabin PI, Lukashevich AI, Fedorova YuE, Sadovskaya EM, Eremeev NF, Krieger TA, Ishchenko AV, Belyaev VD, Uvarov NF, Ulihin AS, Skovorodin IN. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ionics. 2018;322:69–78. doi:10.1016/j.ssi.2018.05.003
Shlyakhtina AV, Pigalskiy KS, Belov DA, Lyskov NV, Kharitonova EP, Kolbanev IV, Borunova AB, Karyagina OK, Sadovskaya EM, Sadykov VA, Eremeev NF. Proton and oxygen ion conductivity in the pyrochlore/fluorite family of Ln2−xCaxScMO7−δ (Ln = La, Sm, Ho, Yb; M = Nb, Ta; x = 0, 0.05, 0.1) niobates and tantalates. Dalton Trans. 2018;47(7):2376–92. doi:10.1039/c7dt03912c
Thoréton V, Hu Y, Pirovano C, Capoen E, Nuns N, Mamede AS, Dezanneau G, Yoo CY, Bouwmeester HJM, Vannier RN. Oxygen transport kinetics of the misfit layered oxide Ca3Co4O9+δ. J Mater Chem A. 2014;2(46):19717–25. doi:10.1039/C4TA02198C
Porotnikova NM, Khodimchuk AV, Zakharov DM, Bogdanovich NM, Osinkin DA. Enhancement of surface exchange and oxygen diffusion of Sr1.95Fe1.4Ni0.1Mo0.5O6–δ oxide determined by two independent isotope exchange methods. Appl Surf Sci. 2023;613:156015. doi:10.1016/j.apsusc.2022.156015
Farlenkov AS, Ananyev MV, Eremin VA, Porotnikova NM, Kurumchin EKh, Melekh B T. Oxygen isotope exchange in doped calcium and barium zirconates. Solid State Ionics. 2016;290:108–15. doi:10.1016/j.ssi.2016.04.015
De Souza RA, Kilner JA, Jeynes C. The application of secondary ion mass spectrometry (SIMS) to the study of high temperature proton conductors (HTPC). Solid State Ionics. 1997;97(1–4):409–19. doi:10.1016/S0167-2738(97)00038-6
Farlenkov AS, Khodimchuk AV, Shevyrev NA, Stroeva AYu, Fetisov AV, Ananyev MV. Oxygen isotope exchange in proton-conducting oxides based on lanthanum scandates. Int J Hydrog Energy. 2019;44(48):26577–88. doi:10.1016/j.ijhydene.2019.08.088
Che W, Wei M, Sang Z, Ou Y, Liu Y, Liu J. Perovskite LaNiO3-δ oxide as an anion-intercalated pseudocapacitor electrode. J Alloys Compd. 2018;731:381–8. doi:10.1016/j.jallcom.2017.10.027
Lu Y, Akbar M, Xia C, Mi Y, Ma L, Wang B, Zhu B. Catalytic membrane with high ion–electron conduction made of strongly correlated perovskite LaNiO3 and Ce0.8Sm0.2O2-δ for fuel cells. J Catal. 2020;386:117–25. doi:10.1016/j.jcat.2020.04.004
Alvarez I, Martı́nez JL, Veiga ML, Pico C. Synthesis, structural characterization, and electronic properties of the LaNi1−xWxO3 (0 ≤ x ≤ 0.25) perovskite-like system. J Solid State Chem. 1996;125(1):47–53. doi:10.1006/jssc.1996.0263
DOI: https://doi.org/10.15826/chimtech.2025.12.2.05
Copyright (c) 2024 Nikita Eremeev, Yulia Bespalko, Ekaterina Sadovskaya, Tamara Krieger, Svetlana Cherepanova, Evgeny Suprun, Arcady Ishchenko, Mikhail Mikhailenko, Mikhail Korobeynikov, Vladislav Sadykov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







