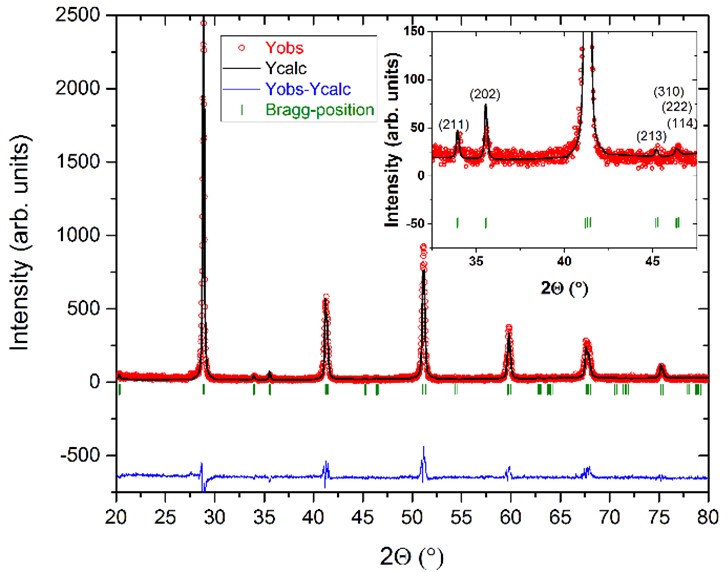
Temperature- and water-induced structural transformations in Ba(Ce0.7Zr0.1Y0.1Yb0.1)O3-δ proton conducting electrolyte
Abstract
Keywords
Full Text:
PDFReferences
Nomura K, Shimada H, Yamaguchi Y, Shin W, Okuyama Y, Mizutani Y. Phase Transitions, Thermal Expansions, Chemical Expansions, and CO2 Resistances of Ba(Ce0.8–xZrxY0.1Yb0.1)O3–δ (x = 0.1, 0.4) Perovskite-Type Proton Conductors. J Electrochem Soc. 2022;169:024516. doi:10.1149/1945-7111/ac5480
Yang L, Wang S, Blinn K, Liu M, Liu Z, Cheng Z, Liu M. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Sci.2009;326:126–129. doi:10.1126/science.1174811
Mirfakhraei B, Ramezanipour F, Paulson S, Birss V, Thangadurai V. Effect of sintering temperature on microstructure, chemical stability, and electrical properties of transition metal or Yb-doped BaZr0.1Ce0.7Y0.1M0.1O3–δ (M=Fe, Ni, Co, and Yb). Fron Energy Res. 2014;2:9. doi:10.3389/fenrg.2014.00009
Wang X, Si X, Li C, Guo X, Cao J. Joining the BaZr0.1Ce0.7Y0.1Yb0.1O3-δ electrolyte to AISI 441 interconnect for protonic ceramic fuel cell applications: interfacial microstructure and long-term stability, ACS Appl Energy Mater. 2021;4:7346–7354. doi:10.1021/acsaem.1c01491
He F, Teng Z, Yang G, Zhou C, Guan D, Chen S, Ran R, Wang W, Zhou W, Shao Z. Manipulating cation nonstoichiometry towards developing better electrolyte for self-humidified dual-ion solid oxide fuel cells. J Power Sources. 2020;460:228105. doi:10.1016/j.jpowsour.2020.228105
Liu Z, Chen Y, Yang G, Yang M, Ji R, Song Y, Ran R, Zhou W, Shao Z. One-pot derived thermodynamically quasi-stable triple conducting nanocomposite as robust bifunctional air electrode for reversible protonic ceramic cells. Appl Catal B Environ. 2022;319:121929. doi:10.1016/j.apcatb.2022.121929
Wan Y, He B, Wang R, Ling Y, Zhao L. Effect of co doping on sinterability and protonic conductivity of BaZr0.1Ce0.7Y0.1Yb0.1O3–δ for protonic ceramic fuel cells. J Power Sources. 2017;347:14–20. doi:10.1016/j.jpowsour.2017.02.049
Yang S, Zhang S, Sun S, Ye X, Wen Z. Lattice incorporation of Cu2+ into the BaCe0.7Zr0.1Y0.1Yb0.1O3-δ electrolyte on boosting its sintering and protonconducting abilities for reversible solid oxide cells. ACS Appl Mater Interfaces. 2018;10:42387–42396. doi:10.1021/acsami.8b15402
Zhang Y, Xie D, Chi B, Pu J, Li J, Yan D. Basic properties of proton conductor BaZr0.1Ce0.7Y0.1Yb0.1O3–δ (BZCYYb) material. Asia-Pacific J Chem Eng. 2019;14(4):e2322. doi:10.1002/apj.2322
Hamze L, Suard E, Joubert O, Quarez E. Synthesis and temperature dependence of the crystal structure of proton conductor BaZr0.1Ce0.7Y0.1Yb0.1O3–δ (BZCYYb1711) by combined neutron and X-ray diffraction. Solid State Ionics. 2024;417:116682. doi:10.1016/j.ssi.2024.116682
Sereda V, Tsvetkov D, Malyshkin D, Ivanov I, Sednev-Lugovets A, Zuev A. Hydration-induced chemical expansion of BaCa(1+y)/3Nb(2−y)/3O3−δ∙xH2O (BCN) and other proton-conducting perovskite oxides. Solid State Ionics. 2020;358:115516. doi:10.1016/j.ssi.2020.115516
DOI: https://doi.org/10.15826/chimtech.2024.11.4.22
Copyright (c) 2024 Ivan Ivanov, Dmitry Tsvetkov, Vladimir Sereda, Dmitry Malyshkin, Anna Sereda, Petr Zakiryanov, Roman Yagovitin, Andrey Zuev

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







