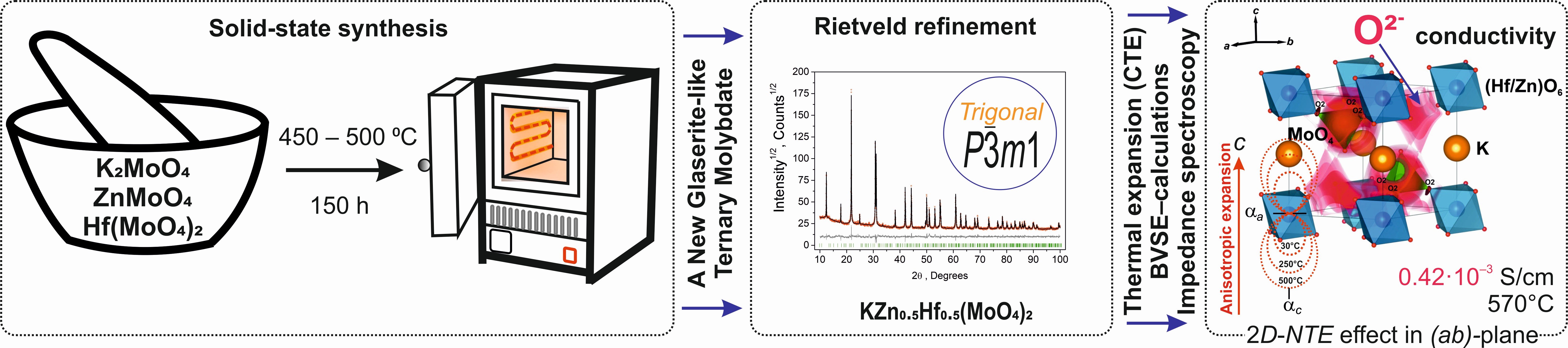
Two-dimensional negative thermal expansion and ionic conductivity of a new glaserite-like ternary molybdate KZn0.5Hf0.5(MoO4)2
Abstract
A new glaserite-like ternary molybdate KZn0.5Hf0.5(MoO4)2 was obtained through a solid-state reaction, and its structure was refined using the Rietveld method. It was found that the compound crystallizes in the trigonal space group P m1 and melts at 656 °C with decomposition. At elevated temperatures, the compound exhibited significant ionic conductivity, reaching 0.39·10–3S/cm at 570 °C with an activation energy Еа = 1.0 eV, with oxygen ions as the probable charge carriers. The infrared spectrum simulated using DFT showed good agreement with experimental data, containing characteristic stretching modes of the MoO4 tetrahedra. The observed negative thermal expansion in the ab plane did not result in a reduction in the volume of the unit cell, with αV = 98∙10–6 °С–1 at 500 °С, indicating that KZn0.5Hf0.5(MoO4)2 can be classified as a material with high thermal expansion properties.
Keywords
Full Text:
PDFReferences
Tsyrenova GD, Pavlova EТ, Solodovnikov SF, Popova NN, Kardash TY, Stefanovich SY, Gudkova IА, Solodovnikova ZA, Lazoryak BI. New ferroelastic K2Sr(MoO4)2: synthesis, phase transitions, crystal and domain structures, ionic conductivity. J Solid State Chem. 2016;237:64–71. doi:10.1016/j.jssc.2016.01.011
Ben NW, Ben RA. Ferroelectric properties and alternative current conduction mechanisms of lithium rubidium molybdate. Ionics. 2019;25:4003–4012. doi:10.1007/s11581-019-02921-w
Stefańska D, Kabański A, Adaszyński M, Ptak M, Lisiecki R, Starościk N, Hanuza J. Broadband near-infrared luminescence properties of Sc2(MoO4)3:Cr3+ molybdates. Spectrochim. Acta Mol Biomol Spectrosc. 2023;296:122699. doi:10.1016/j.saa.2023.122699
Ren H, Li H, Zou Y, Deng H, Peng Z, Ma T, Ding S. Growth and properties of Pr3+-doped NaGd(MoO4)2 single crystal: a promising InGaN laser-diode pumped orange-red laser crystal. J Lumin. 2022;249:119034. doi:10.1016/j.jlumin.2022.119034
Wang J, Luo L, Huang B, He J, Zhang W, Zhao W, Wang J. The preparation and optical properties of novel LiLa(MoO4)2:Sm3+,Eu3+ red phosphor. Mater. 2018;11(2):297. doi:10.3390/ma11020297
Spassky D, Vasil'ev A, Jamal MU, Morozov VA, Lazoryak BI, Redkin BS, Chernenko K, Nagirnyi V. Temperature dependent energy transfer to Eu3+ emission centres in K5Eu(MoO4)4 crystals. CrystEngComm. 2024;26(8):1106–1116. doi:10.1039/D3CE01201H
Posokhova SM, Morozov VA, Zonov EM, Deyneko DV, Spassky DA, Fedyunin FD, Belik AA, Pavlova ET, Vasing AA, Lazoryak BI. K5Yb1−xEux(MoO4)4 phosphors: aperiodic structures and luminescence properties. CrystEngComm. 2023;25;4822–4833. doi:10.1039/D3CE00401E
Solodovnikov SF, Gulyaeva OA, Savina AA, Yudin VN, Buzlukov AL, Solodovnikova ZA, Zolotova ES, Spiridonova TS, Khaikina EG, Stefanovich SY, Medvedeva NI, Baklanova YV, Denisova TA. Molybdates and tungstates of the alluaudite family: crystal chemistry, composition, and ionic mobility. J Struct Chem. 2022;63:1101–1133. doi:10.1134/S0022476622070071
Buzlukov AL, Fedorov DS, Serdtsev AV, Kotova IY, Tyutyunnik AP, Korona DV, Baklanova YV, Ogloblichev VV, Kozhevnikova NM, Denisova TA, Medvedeva NI. Ion mobility in triple sodium molybdates and tungstates with a NASICON structure. J Exp Theor Phys. 2022;134:42–50. doi:10.1134/S1063776122010071
Grossmana VG, Molokeev MS, Bazarov BG, Bazarova JG. Potassium and thallium conductors with a trigonal structure in the M2MoO4–Cr2(MoO4)3–Hf(MoO4)2 (M = K, Tl) systems: Synthesis, structure, and ionic conductivity. J Alloys Compd. 2021;873:159828. doi:10.1016/j.jallcom.2021.159828
Spiridonova TS, Solodovnikov SF, Savina AA, Kadyrova YM, Solodovnikova ZA, Yudin VN, Stefanovich SY, Khaikina EG. New triple molybdate Rb2AgIn(MoO4)3: synthesis, framework crystal structure and ion transport behavior. Acta Crysallogr. С. 2018;74;1603–1609. doi:10.1107/S2053229618014717
Chimitova OD, Bazarov BG, Bazarova JG, Atuchin VV, Azmi V, Sarapulova AE, Mikhailova D, Balachandran G, Fiedler A, Geckle U, Prots Y, Komarek AC, Gavrilova TA, Prosvirin IP, Yang Y, Lin Z, Knapp M, Ehrenberg H. The crystal growth and properties of novel magnetic double molybdate RbFe5(MoO4)7 with mixed Fe3+/Fe2+ states and 1D negative thermal expansion. CrystEngComm. 2021;23:3297–3307. doi:10.1039/D1CE00118C
Nasri R, Larbi T, Amlouk M, Zid MF. Investigation of the physical properties of K2Co2(MoO4)3 for photocatalytic application. J Mater Sci Mater Electron. 2018;29;18372–18379. doi:10.1007/s10854-018-9951-x
Alzakree ARH, Wang CH, Shehbaz M, Wang W, Xu D, Du C, Zhou D. Microwave dielectric properties of (Na0.5Bi0.5)MoO4–BaMoO4 composite ceramics with ultralow sintering temperature. J Am Ceram Soc. 2024;107(11):7452–7459. doi:10.1111/jace.20024
Binish B, Durairaj M, Sabari Girisun TC, Mani Rahulan K. Engineering the nonlinear optical properties of barium molybdate by doping Sn4+ ions for optical limiting device applications. Ceram Int. 2023;49(11);17629–17638. doi:10.1016/j.ceramint.2023.02.129
Klevtsova RF, Bazarova JG, Glinskaya LA, Alekseev VI, Arkhincheeva SI, Bazarov BG, Klevtsov PV, Fedorov KN. Synthesis of ternary potassium, magnesium, and zirconium molybdates. The crystal structure of K5(Mg0.5Zr1.5)⋅(MoO4)6. J Struct Chem. 1994;35:286–290. doi:10.1007/BF02578278
Klevtsova RF, Bazarova JG, Glinskaya LA, Alekseev VI, Arkhincheeva SI, Bazarov BG, Klevtsov PV. Crystal structure investigation of ternary molybdate K(Mg0.5Zr0.5)(MoO4)2. J Struct Chem. 1995;36:809–812. doi:10.1007/BF02579673
Grossman VG, Molokeev MS, Bazarova JG, Bazarov BG. High ionic conductivity of K5-xTlx(Mg0.5Hf1.5)(MoO4)6 (0 ≤ х ≤ 5) solid solutions. Solid State Sci. 2022;134:107027. doi:10.1016/j.solidstatesciences.2022.107027
Aksenov SM, Pavlova ET, Popova NN, Tsyrenova GD, Lazoryak BI. Stoichiometry and topological features of triple molybdates AxByCz(MoO4)n with the heteropolyhedral open MT-frameworks: Synthesis, crystal structure of Rb5{Hf1.5Co0.5(MoO4)6}, and comparative crystal chemistry. Solid State Sci. 2024;151:107525. doi:10.1016/j.solidstatesciences.2024.107525
E.V. Kovtunets, Yu.L. Tushinova, A.V. Logvinova, Ts.T. Bazarova, B.G. Bazarov. Thermal expansion of ternary molybdate K5[Mn0.5Zr1.5](MoO4)6. ESSUTM Bulletin. 2024;3(94):90–97. doi:10.53980/24131997_2024_3_90
Kovtunets EV, Spiridonova TS, Tushinova YL, Logvinova AV, Bazarova TT, Bazarov BG. Thermal expansion and ionic conductivity of K5Pb0.5Zr1.5(MoO4)6. Izvestiya Vuzov. Prikladnaya Khimiya i Biotekhnologiya. 2024;14(4). doi:10.21285/achb.939
Hermanowicz K, Mączka M, Dereń PJ, Hanuza J, Stręk W, Drulis H. Optical properties of chromium(III) in trigonal KAl(MoO4)2 and monoclinic NaAl(MoO4)2 hosts. J Luminescence. 2000;92(1–2):151–159. doi:10.1016/S0022-2313(00)00232-5
Kovtunets E, Tushinova Y, Bazarov B, Bazarova J, Logvinova A, Spiridonova T. A glaserite-like ternary molybdate K(Mg0.5Zr0.5)(MoO4)2: Synthesis, thermal expansion, and ionic conductivity. Solid State Sci. 2024;151:107528. doi:10.1016/j.solidstatesciences.2024.107528
Brik MG, Avram CN. Exchange charge model and analysis of the microscopic crystal field effects in KAl(MoO4)2:Cr3+. J Luminescence. 2011;131(12):2642–2645. doi:10.1016/j.jlumin.2011.06.034
Coelho AA. TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++. J Appl Crystallography. 2018;51:210–218. doi:10.1107/S1600576718000183
Bubnova RS, Firsova VA, Filatov SK. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (theta to tensor-TTT). Glass Phys Chem. 2013;39:347–350. doi:10.1134/S108765961303005X
Chen H, Wong LL, Adams S. SoftBV – a software tool for screening the materials genome of inorganic fast ion conductors. Acta Crystallogr Sect B. 2019;75:18–33. doi:10.1107/S2052520618015718
Gajdoš M, Hummer K, Kresse G, Furthmüller J, Bechstedt F. Linear optical properties in the projector-augmented wave methodology. Phys Rev. 2006;73:045112. doi:10.1103/PhysRevB.73.045112
Perdew JP, Ruzsinszky A, Csonka GI, Vydrov OA, Scuseria GE, Constantin LA, Zhou X, Burke K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys Rev Lett. 2008;100:136406. doi:10.1103/PhysRevLett.100.136406
Togo A, Tanaka I. First-principles phonon calculations in materials science. Scr Mater. 2015;108:1–5. doi:10.1016/j.scriptamat.2015.07.021
Skelton JM, Burton LA, Jackson AJ, Oba J, Parker SC, Walsh A. Lattice dynamics of the tin sulphides SnS2, SnS and Sn2S3: vibrational spectra and thermal transport. Phys Chem Chem Phys. 2017;19:12452–12465. doi:10.1039/C7CP01680H
Fomichev VV, Poloznikova ME, Kondratov OI. Structural features and spectroscopic and energy characteristics of alkali metal molybdates and tungstates. Russ Chem Rev. 1992;61(9):877–888. doi:10.1070/RC1992v061n09ABEH001004
Zhang X, Jiang X, Molokeev MS, Wang N, Liu Y, Lin Zh. Two-dimensional negative thermal expansion in a crystal of LiBO2. Chem Mater. 2022;34(9):4195–4201. doi:10.1021/acs.chemmater.2c00621
Smith AL, Kauric G, Eijck L, Goubitz K, Clavier N, Wallez G, Konings RJM. Structural and thermodynamic study of Cs3Na(MoO4)2: Margin to the safe operation of sodium cooled fast reactors. J Solid State Chem. 2019;269:1–8. doi:10.1016/j.jssc.2018.08.033
Sleight AW. Thermal Contraction. Endeavor. 1995;19(2):64–68. doi:10.1016/0160-9327(95)93586-4
Sleight AW. Compounds that contract on heating. Inorg Chem. 1998;37(12):2854–2860. doi:10.1021/ic980253h
Filatov SK., Krivovichev SV., Bubnova RS. General crystal chemistry: textbook. St. Petersburg: Publishing house of St. Petersburg University. 2018: 276.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica. 1976;32:751–767. doi:10.1107/S0567739476001551
Bazarov BG, Fedorov KN, Bazarova TT, Bazarova JG. Electrical properties of molybdates in the systems M2MoO4–AMoO4–Zr(MoO4)2. Russ J Appl Chem. 2002;75:1026–1028. doi:10.1023/A:1020377905907
DOI: https://doi.org/10.15826/chimtech.2025.12.2.08
Copyright (c) 2024 Evgeniy Kovtunets, Yunna Tushinova, Tatyana Spiridonova, Tsyrendyzhit Bazarova, Alexandra Logvinova, Alexandr Bogdanov, Bair Bazarov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







