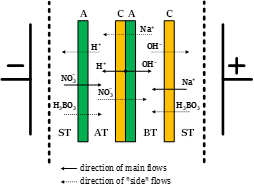
Modified bipolar membrane for electrodialysis processing of highly concentrated sodium nitrate and boric acid solution
Abstract
Keywords
Full Text:
PDFReferences
Myerscough PB. Eds., Nuclear physics and basic technology, Nuclear power generation, Elsevier, 1992. 110 p. doi:10.1016/B978-0-08-040519-3.50008-1
Oto B, Yıldız N, Akdemir F, Kavaz E. Investigation of gamma radiation shielding properties of various ores. Prog Nucl Energy. 2015;85:391–403. doi:10.1016/j.pnucene.2015.07.016
Ivanenko VI, Sedneva TA, Lokshin EP, Kornejkov RI., inventors; Kola Science Center of the Russian Academy of Sciences, assignee. Method for processing liquid waste from nuclear power plants with boron regulation. Russian Federation patent RU 2652978. 2017 Apr 12. Russian.
Dmitriev SA, Lifanov FA, Savkin AE, Lashchenov CM. Handling of the bottom residues of a nuclear power plant. Atomic Energy. 2000;89(5):884–889. doi:10.1023/A:1011338231351
Ministry of Construction, Housing and Utilities of the Russian Federation, Set of rules 127.13330.2017. Landfills for the disposal and burial of toxic industrial waste. Basic design provisions. Approved 2017 Nov 14.
İpekçi D, Kabay N, Bunani S, Altıok E, Arda M, Yoshizuka K, Nishihama S. Application of heterogeneous ion exchange membranes for simultaneous separation and recovery of lithium and boron from aqueous solution with bipolar membrane electrodialysis (EDBM). Desalin. 2020;479:114313. doi:10.1016/j.desal.2020.114313
Figueira M, Reig M, Luis Cortina J, Reza Moradi M, Pihlajamäki A, Valderrama C. Production of boric acid by bipolar membrane electrodialysis: Evaluation of commercial and polyelectrolyte multilayers-coated ion exchange membranes. Sep Purif Technol. 2025;354:129467. doi:10.1016/j.seppur.2024.129467
Kovalev NV, Karpenko TV, Sheldeshov NV, Zabolotsky VI. Electrochemical characteristics of modified heterogeneous bipolar membrane and electromembrane process of nitric acid and sodium hydroxide recuperation from sodium nitrate and boric acid solution. Russ J Electrochem. 2021;57(2):122–133. doi:10.1134/S1023193521020063
Jarma YA, Çermikli E, İpekçi D, Altıok E, Kabay N. Comparison of two electrodialysis stacks having different ion exchange and bipolar membranes for simultaneous separation of boron and lithium from aqueous solution. Desalin. 2021;500:114850. doi:10.1016/j.desal.2020.114850
Hung W-Ch, Horng RS, Tsai Ch-H. Effects of process conditions on simultaneous removal and recovery of boron from boron-laden wastewater using improved bipolar membrane electrodialysis (BMED). J Water Proc Engin. 2022;47:102650. doi:10.1016/j.jwpe.2022.102650
Mauro A. Space charge regions in fixed charge membranes and the associated property of capacitance. Biophys J 1962;2(2):179–198. doi:10.1016/S0006-3495(62)86848-9
Pärnamäe R, Mareev S, Nikonenko V, Melnikov S, Sheldeshov N, Zabolotskii V, Hamelers HVM, Tedesco M. Bipolar membranes: A review on principles, latest developments, and applications. J Memb Sci. 2021;617:118538. doi:10.1016/j.memsci.2020.118538
Bazinet L, Lamarche F, Ippersiel D. Bipolar-membrane electrodialysis: Applications of electrodialysis in the food industry. Trends Food Sci Technol. 1998;9(3):107–113. doi:10.1016/S0924-2244(98)00026-0
Luo Y, Liu Y, Shen J, der Bruggen BV. Application of Bipolar Membrane Electrodialysis in environmental protection and resource recovery: A Review. Membranes. 2022;12(9):829. doi:10.3390/membranes12090829
Wood J, Gifford J, Arba J, Shaw M. Production of ultrapure water by continuous electrodeionization. Desalin. 2010;250(3):973–976. doi:10.1016/j.desal.2009.09.084
Grabowski A, Zhang G, Strathmann H, Eigenberger G. The production of high purity water by continuous electrodeionization with bipolar membranes: Influence of the anion-exchange membrane permselectivity. J Memb Sci. 2006;281(1–2):297–306. doi:10.1016/j.memsci.2006.03.044
Raucq D, Pourcelly G, Gavach C. Production of sulphuric acid and caustic soda from sodium sulphate by electromembrane processes. Comparison between electro-electrodialysis and electrodialysis on bipolar membrane. Desalin. 1993;91(2):163–175. doi:10.1016/0011-9164(93)80055-R
Achoh A, Zabolotsky V, Melnikov S. Conversion of water-organic solution of sodium naphtenates into naphtenic acids and alkali by electrodialysis with bipolar membranes. Sep Purif Technol. 2019;212:929–940. doi:10.1016/j.seppur.2018.12.013
Boyaval P, Seta J, Gavach C. Concentrated propionic acid production by electrodialysis. Enzyme Microb Technol. 1993;15(8):683–686. doi:10.1016/0141-0229(93)90069-E
Niftaliev S, Kozaderova O, Kim K. Application of Bipolar Electrodialysis with Modified Membranes for the Purification of Chromic Wastewater from Galvanic Production. Ecology Industry Russ. 2021;25(10):4–9. doi:10.18412/1816-0395-2021-10-4-9
Chen T, Bi J, Ji Z, Yuan J, Zhao Y. Application of bipolar membrane electrodialysis for simultaneous recovery of high-value acid/alkali from saline wastewater: An in-depth review. Water Res. 2022;226:119274. doi:10.1016/j.watres.2022.119274
Ilhan F, Kabuk HA, Kurt U, Avsar Y, Gonullu MT. Recovery of mixed acid and base from wastewater with bipolar membrane electrodialysis - A case study. Desalin Water Treat. 2016;57(11): 5165–5173. doi:10.1080/19443994.2014.1002012
Ferrari F, Pijuan M, Molenaar S, Duinslaeger N, Sleutels T, Kuntke Ph, Radjenovic J. Ammonia recovery from anaerobic digester centrate using onsite pilot scale bipolar membrane electrodialysis coupled to membrane stripping. Water Res. 2022;218:118504. doi:10.1016/j.watres.2022.118504
Zabolotskii VI, Shel'deshov NV, Gnusin NP. Dissociation of water molecules in systems with ion-exchange membranes. Russ Chem Rev. 1988;57(8):801–808. doi:10.1070/RC1988v057n08ABEH003389
Zabolotckii VI, Gnusin NP, Sheldeshov NV. Vliianie prirody' ionogenny'kh grupp na konstanty' dissotciatcii vody' v bipoliarny'kh ionoobmenny'kh membranakh. Elektrohimiia (in Russian). 1986;22:1676–1679.
Zabolotskii V, Sheldeshov N, Melnikov S. Heterogeneous bipolar membranes and their application in electrodialysis. Desal. 2014;342:183–203. doi:10.1016/j.desal.2013.11.043
Achoh AR, Zabolotsky VI, Lebedev KA, Sharafan MV, Yaroslavtsev AB. Electrochemical properties and selectivity of bilayer ion-exchange membranes in ternary solutions of strong electrolytes. Membranes Membrane Technol. 2021;3(1):52–71. doi:10.1134/S2517751621010029
Zabolotsky VI, Achoh AR, Lebedev KA, Melnikov SS. Permselectivity of bilayered ion-exchange membranes in ternary electrolyte. J Memb Sci. 2020;608:118152. doi:10.1016/j.memsci.2020.118152
Yurova PA, Stenina IA, Yaroslavtsev AB. The effect of the cation-exchange membranes mk-40 modification by perfluorinated sulfopolymer and ceria on their transport properties. Russ J Electrochem. 2020;56(6):528–532. doi:10.1134/S1023193520060154
Zabolotskii V, Sheldeshov N, Melnikov S. Effect of cation-exchange layer thickness on electrochemical and transport characteristics of bipolar membranes. J Appl Electrochem. 2013;43(11):1117–1129. doi:10.1007/s10800-013-0560-3
Zabolotsky VI, Sheldeshov NV, Melnikov SS. inventors; Kuban State University, assignee. Asymmetrical bipolar membrane. Russian Federation patent RU 120373 U1. 2012 Jun 08. Russian.
Peng Z, Cheng Y, Ma X, Hu X, Han Y, Wang H. Thermal effect of the stack in a bipolar membrane electrodialysis: Mechanism, risk, size effect and salt effect. Desalin. 2024;586:117838. doi:10.1016/j.desal.2024.117838
Nemodruk ZK, Karalova AA, Analytical chemistry of boron, Ann Arbor: Ann Arbor-Humphrey Science Publishers, 1969. 236 p.
Edwards JO, Morrison GC, Ross VF, Schultz JW. The structure of the aqueous borate ion. J Am Chem Soc. 1955;77(2):266–268. doi:10.1021/ja01607a005
Zhou Y, Fang C, Fang Y, Zhu F. Polyborates in aqueous borate solution: A Raman and DFT theory investigation. Spectrochim. Acta Part A Mol Biomol Spectrosc. 2011;83(1):82–87. doi:10.1016/j.saa.2011.07.081
Berezina NP, Kononenko NA, Dyomina OA, Gnusin NP. Characterization of ion-exchange membrane materials: Properties vs structure. Adv Colloid Interface Sci. 2008;139(1–2):3–28. doi:10.1016/j.cis.2008.01.002
DOI: https://doi.org/10.15826/chimtech.2025.12.1.15
Copyright (c) 2024 Nazar Romanyuk, Julia Loza, Sergey Alexeevich Loza, Nikita Kovalchuk, Victor Zabolotsky

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







