Synthesis and selective Cu(II) complexation of lower rim substituted thiacalixarenes containing pyrazole fragments
Abstract
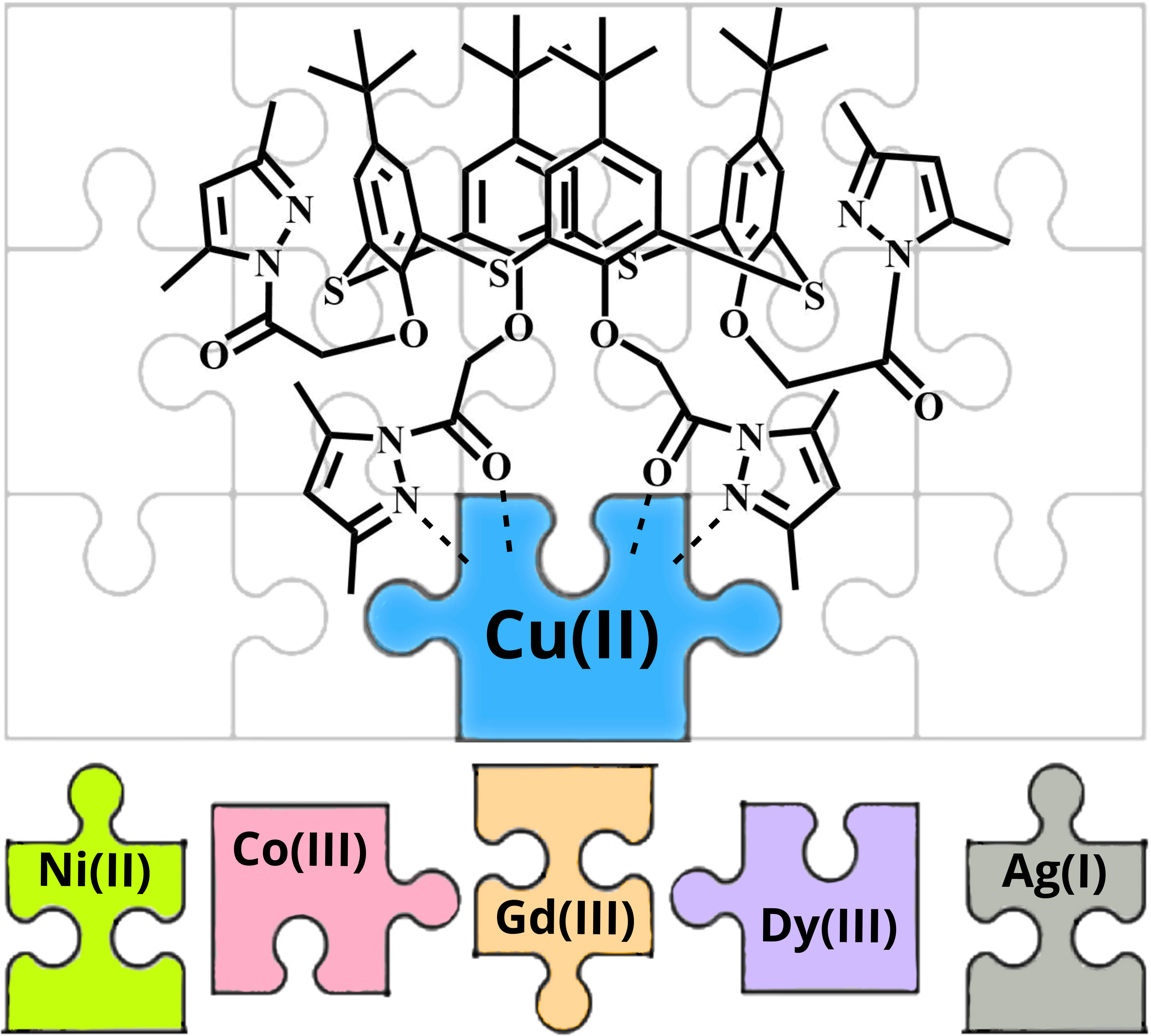
Selective sensors for the detection and quantification of Cu(II) ions are actively used to investigate in vivo conditions in Wilsons and Menkes diseases at the cellular level. Such sensors must have a high specificity for this cation as well as low toxicity. A synthetic approach for the preparation of selective sensors for Cu(II) cations based on p-tert-butylthiacalix[4]arenes modified with 3,5-dimethylpyrazole moieties in cone, partial cone and 1,3-alternate stereoisomeric forms was developed. The obtained compounds were characterized by single-crystal XRD, NMR and IR spectroscopy, MALDI MS, and elemental analysis. All synthesized thiacalixarene compounds formed complexes with Cu(II) with the binding constants in range logK = 4.44–4.63 and 1:1 stoichiometry. The structure of the obtained complexes was studied by NMR spectroscopy and DFT methods. The obtained results can be used to develop selective Cu(II) sensors.
Keywords
Full Text:
PDFReferences
Crisponi G, Nurchi VM, Fanni D, Gerosa C, Nemolato S, Faa G. Copper-related diseases: from chemistry to molecular pathology. Coord Chem Rev. 2010;254(7–8):876–889. doi:10.1016/j.ccr.2009.12.018
Tümer Z, Møller LB. Menkes disease. Eur J Hum Genet. 2010;18(5):511–518. doi:10.1038/ejhg.2009.187
Purchase R. The link between copper and Wilson's Disease. Sci Prog. 2013;96(3):213–223. doi:10.3184/003685013X13712193905878
Ahuja A, Dev K, Tanwar RS, Selwal KK, Tyagi PK. Copper mediated neurological disorder: visions into amyotrophic lateral sclerosis, alzheimer and menkes disease. J Trace Elements Med Biol. 2015;29(1):11–23. doi:10.1016/j.jtemb.2014.05.003
Więcek S, Paprocka J. Disorders of copper Metabolism in Children—A problem too rarely Recognized. Metabolites. 2024;14(1):38. doi:10.3390/metabo14010038
Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer’s disease. JBIC J Biol Inorg Chem. 2010;15(1):61–76. doi:10.1007/s00775-009-0600-y
Bisaglia M, Bubacco L. Copper ions and Parkinson’s Disease: why Is homeostasis So Relevant?. Biomolecules. 2020;10(2):195. doi:10.3390/biom10020195
Koutsouraki E, Michmizos D, Patsi O, Tzartos J, Spilioti M, Arnaoutoglou M, Tsolaki M. A probable role of copper in the comorbidity in Wilson’s and Creutzfeldt-Jakob’s Diseases: a case report. Virol J. 2020;17(1). doi:10.1186/s12985-020-01309-x
Duncan C, White AR. Copper complexes as therapeutic agents. Metallomics. 2011;4(2):127–138. doi:10.1039/c2mt00174h
Gromadzka G, Grycan M, Przybyłkowski AM. Monitoring of copper in wilson Disease. Diagnostics. 2023;13(11):1830. doi:10.3390/diagnostics13111830
Liu H, Li FX, Pi Y, Wang DJ, Hu YJ, Zheng J. Fluorescence quenching study of 2,6-bis(5-(4-methylphenyl)-1- H -pyrazol-3-yl)pyridine with metal ions. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;145:588–593. doi:10.1016/j.saa.2015.03.049
Zhao J, Zhang L, Huang Q, Ma D, Ren T. A near-infrared AIEE fluorescent chemosensor for Cu2+ through an IPT process. Inorg Chim Acta. 2023;555:121579. doi:10.1016/j.ica.2023.121579
Mithra U, Sarveswari, S. A review on pyrazole moieties as organic chemosensors in the detection of cations and anions. Inorg Chim Acta. 2024;569:122118. doi:10.1016/j.ica.2024.122118
Hu Q, Song YF, Wu WN, Zhao XL, Wang Y, Fan YC. A coumarin-pyrazole-based probe for the fluorescence detection of phosgene with high selectivity and sensitivity. Anal Methods. 2023;15(22):2761–2765. doi:10.1039/D3AY00516J
Turones LC, Martins AN, Moreira LKS, Fajemiroye JO, Costa EA. Development of pyrazole derivatives in the management of inflammation. Fundam Clin Pharmacol. 2021;35(2):217–234. doi:10.1111/fcp.12629
Ravindar L, Hasbullah SA, Rakesh KP, Hassan NI. Pyrazole and pyrazoline derivatives as antimalarial agents: A key review. Eur J Pharm Sci. 2023;183:106365. doi:10.1016/j.ejps.2022.106365
Lorthiois E, Gerspacher M, Beyer KS, Vaupel A, Leblanc C, Stringer R, Weiss A, Wilcken R, Guthy DA, Lingel A, Bomio-Confaglia C, Machauer R, Rigollier P, Ottl J, Arz D, Bernet P, Desjonqueres G, Dussauge S, Kazic-Legueux M, Lozac’h MA, Mura C, Sorge M, Todorov M, Warin N, Zink F, Voshol H, Zecri FJ, Sedrani RC, Ostermann N, Brachmann SM, Cotesta S. JDQ443, a structurally Novel, Pyrazole-Based, covalent Inhibitor of KRASG12C for the treatment of solid Tumors. J Med Chem. 2022;65(24):16173–16203. doi:10.1021/acs.jmedchem.2c01438
Shchegolkov EV, Perminova AN, Malkova NA, Kushch SO, Burgart YV, Triandafilova GA, Solodnikov SY, Krasnykh OP, Saloutin VI. Modifications of 4‐Amino‐substituted 5‐Phenyl‐3‐(trifluoromethyl)pyrazoles for the development of new Analgesics. ChemistrySelect. 2023;8(47). doi:10.1002/slct.202303265
dos Santos GC, Martins LM, Bregadiolli BA, Moreno VF, da Silva‐Filho LC, da Silva BHST. Heterocyclic compounds as antiviral drugs: Synthesis, structure–activity relationship and traditional applications. J Heterocycl Chem. 2021;58(12):2226–2260. doi:10.1002/jhet.4349
Hemdan BA, Radwan EK, Rashdan HRM. Design and solvent free synthesis of novel phenazone based molecule for water disinfection and rapid removal of the anionic direct fast blue B2RL dye. J Water Process Eng. 2023;54:103861. doi:10.1016/j.jwpe.2023.103861
Althomali RH, Alamry KA, Hussein MA, Guedes RM. An investigation on the adsorption and removal performance of a carboxymethylcellulose-based 4-aminophenazone@MWCNT nanocomposite against crystal violet and brilliant green dyes. RSC Adv. 2023;13(7):4303–4313. doi:10.1039/D2RA07321H
Silva F, Costa G, Veiga F, Cardoso C, Paiva-Santos AC. Parenteral Ready-to-use Fixed-dose Combinations including NSAIDs with paracetamol or metamizole for multimodal Analgesia—approved Products and Challenges. Pharmaceuticals. 2023;16(8):1084. doi:10.3390/ph16081084
Yushkova EA, Stoikov II, Zhukov AY, Puplampu JB, Rizvanov IK, Antipin IS, Konovalov A. Heteroditopic p-tert-butyl thiacalix[4]arenes for creating supramolecular self-assembles by cascade or commutative mechanisms. RSC Adv. 2012;2(9):3906. doi:10.1039/C2RA01255C
Padnya PL, Andreyko EA, Gorbatova PA, Parfenov VV, Rizvanov IK, Stoikov II. Towards macrocyclic ionic liquids: novel ammonium salts based on tetrasubstituted p-tert-butylthiacalix[4]arenes. RSC Adv. 2017;7(3):1671–1686. doi:10.1039/C6RA24734B
Stoikov II, Yushkova EA, Bukharaev AA, Biziaev DA, Selivanovskaya SY, Chursina MA, Antipin IS, Konovalov AI, Zharov I. Self‐assembly of p‐tert‐butyl thiacalix[4]arenes and metal cations into nanoscale three‐dimensional particles. J Phys Org Chem. 2012;25(12):1177–1185. doi:10.1002/poc.2981
Yakimova L, Kunafina A, Nugmanova A, Padnya P, Voloshina A, Petrov K, Stoikov I. Structure–activity Relationship of the Thiacalix[4]arenes family with sulfobetaine Fragments: Self-assembly and cytotoxic Effect against cancer Cell Lines. Molecules. 2022;27(4):1364. doi:10.3390/molecules27041364
Padnya PL, Terenteva OS, Akhmedov AA, Iksanova AG, Shtyrlin NV, Nikitina EV, Krylova ES, Shtyrlin YG, Stoikov II. Thiacalixarene based quaternary ammonium salts as promising antibacterial agents. Bioorg Med Chem. 2021;29:115905. doi:10.1016/j.bmc.2020.115905
Shiabiev I, Pysin D, Akhmedov A, Babaeva O, Babaev V, Lyubina A, Voloshina A, Petrov K, Padnya P, Stoikov I. Towards antibacterial Agents: synthesis and biological Activity of multivalent Amide derivatives of Thiacalix[4]arene with hydroxyl and amine Groups. Pharmaceutics. 2023;15(12):2731. doi:10.3390/pharmaceutics15122731
Nazarova A, Shiabiev I, Shibaeva K, Mostovaya O, Mukhametzyanov T, Khannanov A, Evtugyn V, Zelenikhin P, Shi X, Shen M, Padnya P, Stoikov I. Thiacalixarene carboxylic Acid derivatives as inhibitors of lysozyme Fibrillation. Int J Mol Sci. 2024;25(9):4721. doi:10.3390/ijms25094721
Alekseeva EA, Krasnoshchekaya SP, Gren' AI. Synthesis of p-tert-Butylcalix[4]arene Derivatives Containing Acylpyrazole Fragments. Russ J Gen Chem. 2002;72(1):157–159. doi:10.1023/A:1015386505286
Muravev AA, Voloshina AD, Sapunova AS, Gabdrakhmanova FB, Lenina OA, Petrov KA, Shityakov S, Skorb EV, Solovieva SE, Antipin IS. Calix[4]arene–pyrazole conjugates as potential cancer therapeutics. Bioorg Chem. 2023;139:106742. doi:10.1016/j.bioorg.2023.106742
Chen YJ, Chen MY, Lee KT, Shen LC, Hung HC, Niu HC, Chung WS. 1,3-alternate Calix[4]arene functionalized With pyrazole and triazole Ligands as a highly Selective fluorescent Sensor for Hg2+ and Ag+ Ions. Front Chem. 2020;8. doi:doi.org/10.3389/fchem.2020.593261
Abdou A, Omran OA, Al-Fahemi JH, Jassas RS, Al-Rooqi MM, Hussein EM, Moussa Z, Ahmed SA. Lower rim thiacalixarenes derivatives incorporating multiple coordinating carbonyl groups: Synthesis, characterization, ion-responsive ability and DFT computational analysis. J Mol Struct. 2023;1293:136264. doi:10.1016/j.molstruc.2023.136264
KILIÇ HURYEAKDA, GRAF ERNEST, HOSSEİNİ MRWAS, KYRİTSAKAS NATHALE. New 2-D silver(I) coordination network constructed from thiomethyl group-substituted p-tert-butylthiacalix[4]arene. Turk J Chem. 2022;46(5):1541–1547. doi:10.55730/1300-0527.3459
Fang Y, Xie WX, Han K, Zhou K, Kang L, Shi J, Chen BK, Bi YF. Diphosphine modified copper(I)-thiacalixarene supramolecular structure for effective photocurrent response and photodegradation of methylene blue. Polyhedron. 2022;222:115934. doi:10.1016/j.poly.2022.115934
Zhou Z, Xu L, Zhao G, Zhou K, Chen B, Bi Y. An ultra-stable CuI12 cluster built from a CuI6 precursor sandwiched by two CuI3-thiacalixarene units for efficient photothermal conversion. Inorg Chem Front. 2023;10(11):3230–3236. doi:10.1039/D3QI00479A
Wang Z, Su HF, Gong YW, Qu QP, Bi YF, Tung CH, Sun D, Zheng LS. A hierarchically assembled 88-nuclei silver-thiacalix[4]arene nanocluster. Nat Commun. 2020;11(1). doi:10.1038/s41467-019-13682-5
Takagiri Y, Ikuta T, Maehashi K. Selective detection of Cu2+ ions by immobilizing Thiacalix[4]arene on graphene Field-effect Transistors. ACS Omega. 2020;5(1):877–881. doi:10.1021/acsomega.9b03821
Evtugyn GA, Stoikov II, Beljyakova SV, Shamagsumova RV, Stoikova EE, Zhukov AY, Antipin IS, Budnikov HC. Ag selective electrode based on glassy carbon electrode covered with polyaniline and thiacalix[4]arene as neutral carrier. Talanta. 2007;71(4):1720–1727. doi:10.1016/j.talanta.2006.08.004
Mostovaya O, Padnya P, Shiabiev I, Mukhametzyanov T, Stoikov I. PAMAM-calix-dendrimers: synthesis and thiacalixarene Conformation effect on DNA Binding. Int J Mol Sci. 2021;22(21):11901. doi:10.3390/ijms222111901
Subashini G, Saravanan A, Shyamsivappan S, Arasakumar T, Mahalingam V, Shankar R, Mohan PS. A versatile “on-off-on” quinoline pyrazoline hybrid for sequential detection of Cu2+ and S− ions towards bio imaging and tannery effluent monitoring. Inorg Chim Acta. 2018;483:173–179. doi:10.1016/j.ica.2018.08.012
Nguyen VA, Vu TNA, Polyanskaya N, Utenyshev A, Shilov G, Vasil'eva M, Anh Tien N, Kovalchukova O. Structure and properties of some S-containing azo-derivatives of 5-pyrazolone and their Cu(II), Co(II), and Ni(II) metal complexes. Inorg Chem Commun. 2023;158:111648. doi:10.1016/j.inoche.2023.111648
Radnović ND, Štetin N, Radanović MM, Borišev I, Rodić MV, Jaćimović K, Barta Holló B. Two isomers of a novel Ag(I) complex with Pyrazole-type Ligand—Synthesis, Structural, Thermal, and antioxidative Characterization. Inorg. 2023;12(1):4. doi:10.3390/inorganics12010004
Stoikov II, Yushkova EA, Zharov I, Antipin IS, Konovalov AI. Supramolecular self-assemblies of stereoisomers of p-tert-butyl thiacalix[4]arenes functionalized with hydrazide groups at the lower rim with some metal cations. Tetrahedron. 2009;65(34):7109–7114. doi:10.1016/j.tet.2009.06.045
Hoover JM, DiPasquale A, Mayer JM, Michael FE. Platinum-catalyzed Intramolecular Hydrohydrazination: evidence for alkene Insertion into a Pt−N Bond. J Am Chem Soc. 2010;132(14):5043–5053. doi:10.1021/ja906563z
Hashem HE, Ahmad S, Kumer A, Bakri YE. In silico and in vitro prediction of new synthesized N-heterocyclic compounds as anti-SARS-CoV-2. Sci Rep. 2024;14(1). doi:10.1038/s41598-024-51443-7
Mohamed MA, Ali A, Ali A, Afzal O, Ahsan MF, Alamri MA, Alossaimi MA, Altamimi ASA, Salahuddin, Ahsan MJ. Targeting EGFR by newer 1-(3,5-Bis((E)-4 hydroxy-3-methoxystyryl)-1H-pyrazol-1-yl)-2-((substituted phenyl)amino)ethan-1-one analogues for the treatment of Cancer: Synthesis, In-vitro and In-silico Studies. J Mol Struct. 2024;1315:138826. doi:10.1016/j.molstruc.2024.138826
Staab H.;Bauer H.;Schneider K. Azolides in organic Synthesis and Biochemistry. 1st ed. Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany. 27–79. doi:10.1002/3527600833
Bindfit. Available online: http://supramolecular.org (accessed on 20 September 2024)
Gu YQ, Shen WY, Zhou Y, Chen SF, Mi Y, Long BF, Young DJ, Hu FL. A pyrazolopyrimidine based fluorescent probe for the detection of Cu2+ and Ni2+ and its application in living cells. Spectrochim Acta Part A Mol Biomol Spectrosc. 2019;209:141–149. doi:10.1016/j.saa.2018.10.030
Costa AI, Barata PD, Fialho CB, Prata JV. Highly sensitive and selective Fluorescent probes for Cu(II) detection Based on Calix[4]arene-oxacyclophane Architectures. Molecules. 2020;25(10):2456. doi:10.3390/molecules25102456
Padnya P, Shibaeva K, Arsenyev M, Baryshnikova S, Terenteva O, Shiabiev I, Khannanov A, Boldyrev A, Gerasimov A, Grishaev D, Shtyrlin Y, Stoikov I. Catechol-containing Schiff bases on Thiacalixarene: Synthesis, copper (II) Recognition, and formation of Organic-inorganic Copper-based Materials. Molecules. 2021;26(8):2334. doi:10.3390/molecules26082334
Qazi MA, Ocak, Ocak M, Memon S. An excellent copper selective chemosensor based on calix[4]arene framework. Anal Chim Acta. 2013;761:157–168. doi:10.1016/j.aca.2012.11.026
Pandey R, Kumar A, Xu Q, Pandey DS. Zinc(ii), Cu(II) and cadmium(ii) complexes as fluorescent chemosensors for cations. Dalton Trans. 2019;49(3):542–568. doi:10.1039/C9DT03017D
Ishihara K, Nishimura K, Yamakawa K. Enantio‐ and Site‐selective α‐fluorination of N‐acyl 3,5‐dimethylpyrazoles Catalyzed by chiral π–CuII Complexes. Angew Chem Int Ed. 2020;59(40):17641–17647. doi:10.1002/anie.202007403
Ahmed MA, Zhernakov MA, Gilyazetdinov EM, Bukharov MS, Islamov DR, Usachev KS, Klimovitskii AE, Serov NY, Burilov VA, Shtyrlin VG. Complexes of NiII, CoII, ZnII, and CuII with promising Anti-tuberculosis Drug: Solid-state Structures and DFT Calculations. Inorg. 2023;11(4):167. doi:10.3390/inorganics11040167
Serov NY, Shtyrlin VG, Bukharov MS, Ermolaev AV, Gilyazetdinov EM, Urazaeva KV, Rodionov AA. Complex structures, formation thermodynamics and substitution reaction kinetics in the Cu(II) – glycylglycyl-l-tyrosine – l/d-histidine systems. Polyhedron. 2022;228:116176. doi:10.1016/j.poly.2022.116176
Shtyrlin VG, Serov NY, Bukharov MS, Gilyazetdinov EM, Zhernakov MA, Ahmed MA, Garifzyanov AR, Mirzayanov II, Ermolaev AV, Aksenin NS, Urazaeva KV, Zakharov AV. Stereoselective effects, formation thermodynamics, substitution reaction kinetics, and structures of transition metal complexes with bioligands and aromatic N-donors. Russ Chem Bull. 2023;72(7):1485–1498. doi:10.1007/s11172-023-3926-7
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands;the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J Chem Soc Dalton Trans. 2004;7:1349–1356. doi:10.1039/DT9840001349
Shtyrlin VG, Zyavkina YI, Ilakin VS, Garipov RR, Zakharov AV. Structure, stability, and ligand exchange of Cu(II) complexes with oxidized glutathione. J Inorg Biochem. 2005;99(6):1335–1346. doi:10.1016/j.jinorgbio.2005.03.008
Powell DH, Helm L, Merbach AE. 17O nuclear magnetic resonance in aqueous solutions of Cu2+: the combined effect of Jahn–Teller inversion and solvent exchange on relaxation rates. J Chem Phys. 1991;95(12):9258–9265. doi:10.1063/1.461206
Bukharov MS, Shtyrlin VG, Mukhtarov AS, Mamin GV, Stapf S, Mattea C, Krutikov AA, Il'in AN, Serov NY. Study of structural and dynamic characteristics of Cu(II) amino acid complexes in solutions by combined EPR and NMR relaxation methods. Phys Chem Chem Phys. 2014;16(20):9411. doi:10.1039/c4cp00255e
Neese F. The ORCA program system. WIREs Comput Mol Sci. 2012;2(1):73–78. doi:10.1002/wcms.81
Kohn W, Becke AD, Parr RG. Density functional Theory of electronic Structure. J Phys Chem. 1996;100(31):12974–12980. doi:10.1021/jp960669l
Becke AD. Density-functional thermochemistry. III. the role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG. Development of the Colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 2002;37(2):785–789. doi:10.1103/PhysRevB.37.785
Schäfer A, Huber C, Ahlrichs R. Fully optimized contracted gaussian basis sets of triple zeta valence quality for atoms li to Kr. J Chem Phys. 1994;100(8):5829–5835. doi:10.1063/1.467146
Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys. 2005;7(18):3297. doi:10.1039/B508541A
Weigend F, Häser M, Patzelt H, Ahlrichs R. RI-MP2: optimized auxiliary basis sets and demonstration of efficiency. Chem Phys Lett. 1998;294(1–3):143–152. doi:10.1016/S0009-2614(98)00862-8
Cossi M, Rega N, Scalmani G, Barone V. Energies, structures, and electronic properties of molecules in solution with the C‐PCM solvation model. J Comput Chem. 2003;24(6):669–681. doi:10.1002/jcc.10189
Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurateab initioparametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys. 2010;132(15):1. doi:10.1063/1.3382344
Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J Comput Chem. 2011;32(7):1456–1465. doi:10.1002/jcc.21759
Sheldrick GM. SHELXT– integrated space-group and crystal-structure determination. Acta Crystallogr Sect Adv. 2015;71(1):3–8. doi:10.1107/S2053273314026370
Sheldrick GM. A short history of SHELX. Acta Crystallogr Sect Crystallogr. 2008;64(1):112–122. doi:10.1107/S0108767307043930
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J. Mercury: visualization and analysis of crystal structures. J Appl Crystallogr. 2006;39(3):453–457. doi:10.1107/S002188980600731X
Wang Y, Lu X, Shi J, Xu J, Wang F, Yang X, Yu G, Liu Z, Li C, Dai A, Zhao Y, Wu J. Synthesis and larvicidal activity of 1,3,4-oxadiazole derivatives containing a 3-chloropyridin-2-yl-1H-pyrazole scaffold. Monatshefte für Chem. Chem Mon. 2018;149(3):611–623. doi:10.1007/s00706-017-2060-3
DOI: https://doi.org/10.15826/chimtech.2024.11.4.18
Copyright (c) 2024 Valeriy Kalinin, Ekaterina Rasperetikhina, Mikhail Bukharov, Ksenia Shibaeva, Daut Islamov, Olga Babaeva, Ivan Stoikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







