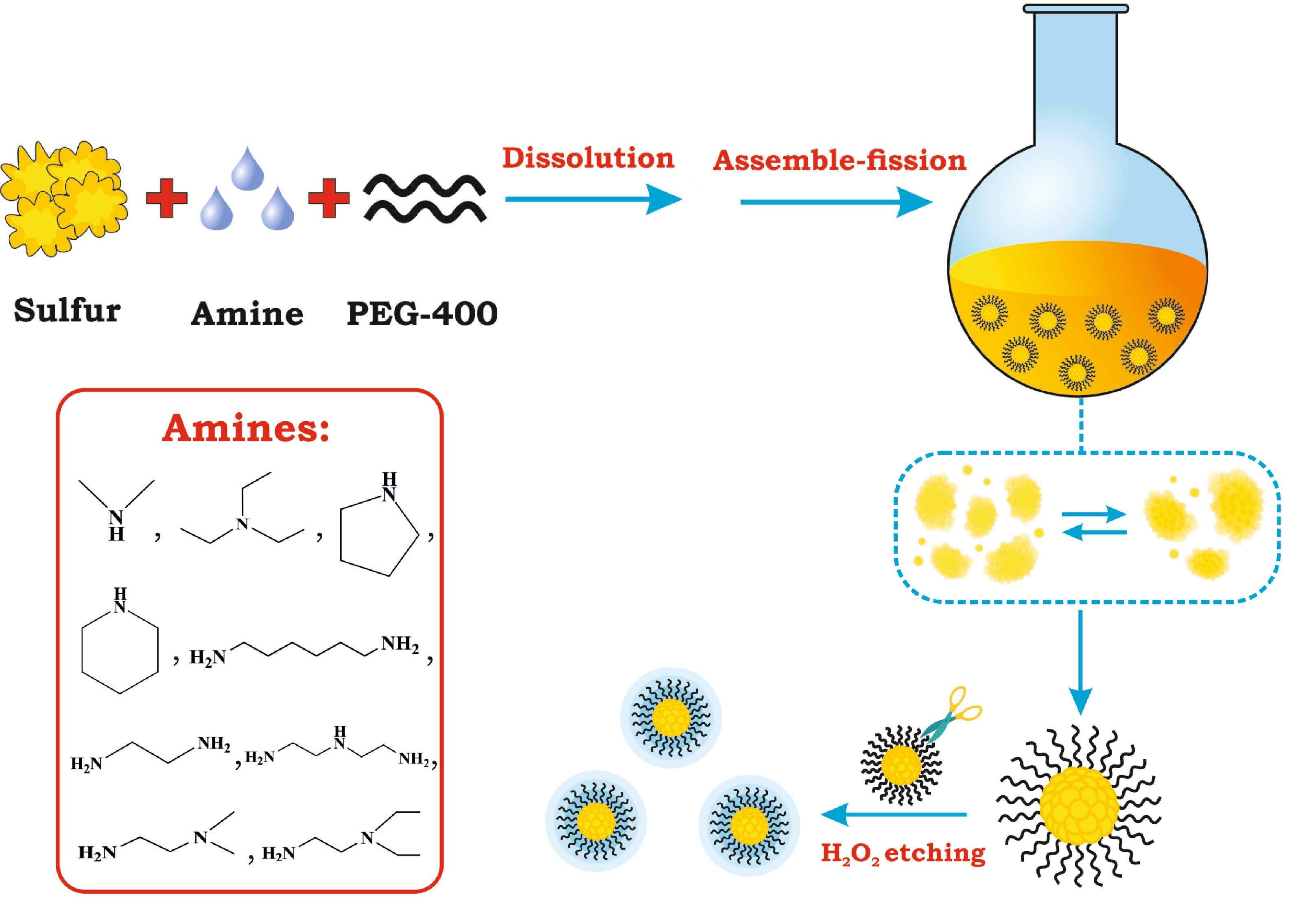
One-step synthesis of sulfur quantum dots in the presence of primary, secondary, and tertiary amines
Abstract
Keywords
Full Text:
PDFReferences
Bera D, Qian L, Tseng TK, Holloway PH. Quantum dots and their multimodal applications: a review. Materi-als. 2010; 3(4): 2260-2345. doi: 10.3390/ma3042260
García de Arquer FP, Talapin DV, Klimov VI, Arakawa Y, Bayer M, Sargent EH. Semiconductor quantum dots: Technological progress and future challenges. Science. 2021; 373(6555): eaaz8541. doi: 10.1126/science.aaz8541
Azam N., Najabat Ali M., Javaid Khan T. Carbon quan-tum dots for biomedical applications: review and analysis. Front. Mater. 2021; 8: 700403. doi: 10.3389/fmats.2021.700403
Truong KT, Pham TH, Tran KV. The impact of dime-thylformamide on the synthesis of graphene quantum dots derived from graphene oxide. Chim. tech. acta. 2023; 10 (4). doi: 10.15826/chimtech.2023.10.4.05
Gidwani B., Sahu V., Shukla SS, Pandey R., Joshi V., Jain VK., Vyas A. Quantum dots: Prospectives, toxici-ty, advances and applications. J. Drug. Deliv. Sci. Technol. 2021; 61: 102308. doi: 10.1016/j.jddst.2020.102308
Drbohlavova J, Adam V, Kizek R, Hubalek J. Quantum dots—characterization, preparation and usage in bio-logical systems. Int. J. Mol. Sci. 2009; 10(2): 656-673. doi: 10.3390/ijms10020656
Hu L, Zhong H, He Z. Toxicity evaluation of cadmium-containing quantum dots: A review of optimizing phys-icochemical properties to diminish toxicity. Colloids Surf. B Biointerfaces. 2021; 200: 111609. doi: 10.1016/j.colsurfb.2021.111609
Nikazar S, Sivasankarapillai VS, Rahdar A, Gasmi S, Anumol PS, Shanavas MS. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020; 12: 703-718. doi: 10.1007/s12551-020-00653-0
Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, & Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007; 28(31): 4717-4732. doi: 10.1016/j.biomaterials.2007.07.014
Shen L, Wang H, Liu S, Bai Z, Zhang S, Zhang X, Zhang C. Assembling of sulfur quantum dots in fission of sub-limed sulfur. J. Am. Chem. Soc. 2018; 140(25): 7878-7884. doi: 10.1021/jacs.8b02792
Pal A, Arshad F, Sk MP. Emergence of sulfur quantum dots: Unfolding their synthesis, properties, and appli-cations. Adv. Colloid Interface Sci. 2020; 285: 102274. doi: 10.1016/j.cis.2020.102274
Gao P, Wang G, Zhou L. Luminescent sulfur quantum dots: Synthesis, properties and potential applications. ChemPhotoChem. 2020; 4(11): 5235-5244. doi: 10.1002/cptc.202000158
Kumar JV, Tammina SK, Rhim JW. One-step hydro-thermal synthesis of sulfur quantum dots for detection of Hg2+ ions and latent fingerprints. Colloids Surf. A: Physicochem. Eng. Asp. 2024; 690: 133682. doi: 10.1016/j.colsurfa.2024.133682
Wang H, Wang Z, Xiong Y, Kershaw SV, Li T, Wang Y, Zhai Y, Rogach AL. Hydrogen peroxide assisted syn-thesis of highly luminescent sulfur quantum dots. An-gew. Chem., Int. Ed. Engl. 2019; 131(21): 7114-7118. doi: 10.1002/ange.201902344
Huang Y, Liu Y, Fu N, Huang Q, Zhang H. Advances in the synthesis and properties of sulfur quantum dots for food safety detection and antibacterial applica-tions. Food Chemistry, 2024: 141055. doi: 10.1016/j.foodchem.2024.141055
DOI: https://doi.org/10.15826/chimtech.2025.12.1.05
Copyright (c) 2024 Dmitriy Shurpik, Inna Tanaeva, Olga Mostovaya, Ivan Stoikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







