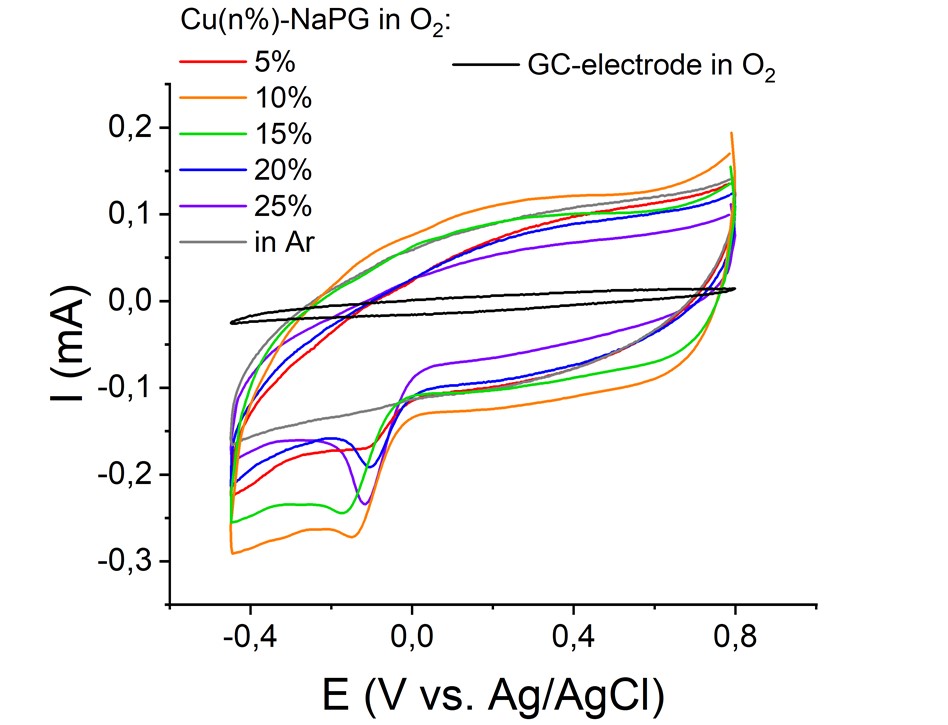
Electrocatalytic activity of sodium copper pectates in oxygen reduction reaction in fuel cells
Abstract
Keywords
Full Text:
PDFReferences
Nieva Lobos ML, Sieben JM, Moyano EL. Softwood Kraft Pulp-Derived Carbon-Supported PtNi Catalysts for the Electrooxidation of Ethanol. Front Mater. 2020;7:588399. doi:10.3389/fmats.2020.588399
Kirik N, Rabchevskii E, Shishkina N, Solovyov L, Anshits A. Composite catalysts based on the CaO‒Fe2O3 system for the oxidative conversion of methane. Chimica Techno Acta. 2024;11(4):202411401. doi:10.15826/chimtech.2024.11.4.01
Sadykov V, Eremeev N, Sadovskaya E, Zhulanova T, Pikalov S, Fedorova Y, Pikalova E. Impact of calcium and copper co-doping on the oxygen transport of layered nickelates: a case study of Pr1.6Ca0.4Ni1–yCuyO4+δ and a comparative analysis. Chimica Techno Acta. 2024;11(4):202411411. doi:10.15826/chimtech.2024.11.4.11
Jamil A, Rafiq S, Iqbal T, Khan HAA, Khan HM, Azeem B, Mustafa MZ, Hanbazazah AS. Current status and future perspectives of proton exchange membranes for hydrogen fuel cells. Chemosphere. 2022;303:135204. doi:10.1016/j.chemosphere.2022.135204
Lohse-Busch H, Stutenberg K, Duoba M, Liu X, Elgowainy A, Wang M, Wallner T, Richard B, Christenson M. Automotive fuel cell stack and system efficiency and fuel consumption based on vehicle testing on a chassis dynamometer at minus 18 C to positive 35 C temperatures. Int J Hydrogen Energy. 2020;45(1):861–72. doi:10.1016/j.ijhydene.2019.10.150
Dyantyi N, Parsons A, Sita C, Pasupathi S. PEMFC for aeronautic applications: A review on the durability aspects. Open Engineering. 2017;7(1):287–302. doi:10.1515/eng-2017-0035
Rodríguez-Castellanos A, Díaz-Bernabé JL, Citalán-Cigarroa S, Solorza-Feria O. Development and applications of portable systems based on conventional PEM fuel cells. Portable Hydrogen Energy Syst. 2018;91–106. doi:10.1016/B978-0-12-813128-2.00006-1
Zaman S, Huang L, Douka AI, Yang H, You B, Xia BY. Oxygen reduction electrocatalysts toward practical fuel cells: progress and perspectives. Angew Chem. 2021;133(33):17976–96. doi:10.1002/ange.202016977
Huang L, Zaman S, Tian X, Wang Z, Fang W, Xia BY. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc Chem Res. 2021;54(2):311–22. doi:10.1021/acs.accounts.0c00488
Wang X, Li Z, Qu Y, Yuan T, Wang W, Wu Y, Li Y. Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chem. 2019;5(6):1486–511. doi:10.1016/j.chempr.2019.03.002
Ioroi T, Siroma Z, Yamazaki SI, Yasuda K. Electrocatalysts for PEM fuel cells. Adv Energy Mater. 2019;9(23):1801284. doi:10.1002/aenm.201801284
Zhu E, Wu M, Xu H, Peng B, Liu Z, Huang Y, Li Y. Stability of Platinum‐Group‐Metal‐Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. Adv Funct Mater. 2022;32(30):2203883. doi:10.1002/adfm.202203883
Nie Y, Li L, Wei Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem Soc Rev. 2015;44(8):2168–201. doi:10.1039/C4CS00484A
Du L, Prabhakaran V, Xie X, Park S, Wang Y, Shao Y. Low‐PGM and PGM‐free catalysts for proton exchange membrane fuel cells: stability challenges and material solutions. Adv Mater. 2021;33(6):1908232. doi:10.1002/adma.201908232
Escudero-Escribano M, Malacrida P, Hansen MH, Vej-Hansen UG, Velázquez-Palenzuela A, Tripkovic V, Schiøtz J, Rossmeisl J, Stephens IEL, Chorkendorff I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Sci. 2016;352(6281):73–6. doi:10.1126/science.aad8892
Greeley J, Stephens IEL, Bondarenko AS, Johansson TP, Hansen HA, Jaramillo TF, Rossmeisl J, Chorkendorff I, Nørskov JK. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat Chem. 2009;1(7):552-56. doi:10.1038/nchem.367
Chung DY, Jun SW, Yoon G, Kwon SG, Shin DY, Seo P, Yoo JM, Shin H, Chung YH, Kim H, Mun BS, Lee KS, Lee NS, Yoo SJ, Lim DH, Kang K, Sung YE, Hyeon T. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J Am Chem Soc. 2015;137(49):15478–85. doi:10.1021/jacs.5b09653
Wang XX, Sokolowski J, Liu H, Wu G. Pt alloy oxygen-reduction electrocatalysts: Synthesis, structure, and property. Chin J Catal. 2020;41(5):739–55. doi:10.1016/S1872-2067(19)63407-8
Stamenkovic VR, Mun BS, Arenz M, Mayrhofer KJ, Lucas CA, Wang G, Ross PN, Markovic NM. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat Mater. 2007;6(3):241–47. doi:10.1038/nmat1840
Han B, Carlton CE, Kongkanand A, Kukreja RS, Theobald BR, Gan L, O'Malley R, Strasser P, Wagner FT, Shao-Horn Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ Sci. 2015;8(1):258–66. doi:10.1039/C4EE02144D
Sneed BT, Cullen DA, Mukundan R, Borup RL, More KL. PtCo cathode catalyst morphological and compositional changes after PEM fuel cell accelerated stress testing. J Electrochem Soc. 2018;165(6):F3078. doi:10.1149/2.0091806jes
Ball SC, Hudson SL, Leung JH, Russell AE, Thompsett D, Theobald BR. Mechanisms of activity loss in PtCo alloy systems. ECS Trans. 2007;11(1):1247. doi:10.1149/1.2781038
Todoroki N, Kawamura R, Asano M, Sasakawa R, Takahashi S, Wadayama T. Alloy-composition-dependent oxygen reduction reaction activity and electrochemical stability of Pt-based bimetallic systems: a model electrocatalyst study of Pt/PtxNi100− x(111). Phys Chem Chem Phys. 2018;20(17):11994–12004. doi:10.1039/C8CP01217B
Thanasilp S, Hunsom M. Effect of Pt: Pd atomic ratio in Pt–Pd/C electrocatalyst-coated membrane on the electrocatalytic activity of ORR in PEM fuel cells. Renewable Energy. 2011;36(6):1795–801. doi:10.1016/j.renene.2010.10.024
Stamenkovic VR, Fowler B, Mun BS, Wang G, Ross PN, Lucas CA, Markovic NM. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science. 2007;315(5811):493–97. doi:10.1126/science.1135941
Arán-Ais RM, Dionigi F, Merzdorf T, Gocyla M, Heggen M, Dunin-Borkowski RE, Gliech M, Solla-Gullón J, Herrero E, Feliu JM, Strasser P. Elemental anisotropic growth and atomic-scale structure of shape-controlled octahedral Pt–Ni–Co alloy nanocatalysts. Nano Lett. 2015;15(11):7473–80. doi:10.1021/acs.nanolett.5b03057
Huang X, Zhao Z, Cao L, Chen Y, Zhu E, Lin Z, Li M, Yan A, Zettl A, Wang YM, Duan X, Mueller T, Huang Y. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science. 2015;348(6240):1230-34. doi:10.1126/science.aaa8765
Bezerra CW, Zhang L, Lee K, Liu H, Marques AL, Marques EP, Wang H, Zhang J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim Acta. 2008;53(15):4937–51. doi:10.1016/j.electacta.2008.02.012
Li H, Wen Y, Jiang M, Yao Y, Zhou H, Huang Z, Li J, Jiao S, Kuang Y, Luo S. Understanding of neighboring Fe‐N4‐C and Co‐N4‐C dual active centers for oxygen reduction reaction. Adv Funct Mater. 2021;31(22):2011289. doi:10.1002/adfm.202011289
Sa YJ, Seo DJ, Woo J, Lim JT, Cheon JY, Yang SY, Lee JM, Kang D, Shin TJ, Shin HS, Jeong HY, Kim CS, Kim MG, Kim TY, Joo SH. A general approach to preferential formation of active Fe–N x sites in Fe–N/C electrocatalysts for efficient oxygen reduction reaction. J Am Chem Soc. 2016;138(45):15046–56. doi:10.1021/jacs.6b09470
Hossen MM, Artyushkova K, Atanassov P, Serov A. Synthesis and characterization of high performing Fe-NC catalyst for oxygen reduction reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J Power Sources. 2018;375:214–21. doi:10.1016/j.jpowsour.2017.08.036
Wang R, Zhang P, Wang Y, Wang Y, Zaghib K, Zhou Z. ZIF-derived Co–N–C ORR catalyst with high performance in proton exchange membrane fuel cells. Prog in Nat Sci Mater Int. 2020;30(6):855–60. doi:10.1016/j.pnsc.2020.09.010
Im K, Jang JH, Heo J, Kim D, Lee KS, Lim HK, Kim J, Yoo SJ. Design of Co-NC as efficient electrocatalyst: The unique structure and active site for remarkable durability of proton exchange membrane fuel cells. Appl Catal B Environ. 2022;308:121220. doi:10.1016/j.apcatb.2022.121220
Stracensky T, Jiao L, Sun Q, Liu E, Yang F, Zhong S, Cullen DA, Myers DJ, Kropf AJ, Jia Q, Mukerjee S, Xu H. Bypassing Formation of Oxide Intermediate via Chemical Vapor Deposition for the Synthesis of an Mn-NC Catalyst with Improved ORR Activity. ACS Catal. 2023;13(22):14782–91. doi:10.1021/acscatal.3c01982
Wen X, Yu C, Yan B, Zhang X, Liu B, Xie H, Shen PK, Tian ZQ. Morphological and microstructural engineering of Mn-NC with strengthened Mn-N bond for efficient electrochemical oxygen reduction reaction. Chem Eng J. 2023;475:146135. doi:10.1016/j.cej.2023.146135
Yang L, Cheng D, Xu H, Zeng X, Wan X, Shui J, Xiang Z, Cao D. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc Nat Acad Sci. 2018;115(26):6626–31. doi:10.1073/pnas.1800771115
Han Y, Wang YG, Chen W, Xu R, Zheng L, Zhang J, Luo J, Shen RA, Zhu Y, Cheong WC, Chen C, Peng Q, Wang D, Li Y. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction. J Am Chem Soc. 2017;139(48):17269–72. doi:10.1021/jacs.7b10194
Li J, Chen M, Cullen DA, Hwang S, Wang M, Li B, Liu K, Karakalos S, Lucero M, Zhang H, Lei C, Xu H, Sterbinsky GE, Feng Z, Su D, More KL, Wang G, Wang Z, Wu G. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat Catal. 2018;1(12):935–945. doi:10.1038/s41929-018-0164-8
Yan Y, Cheng H, Qu Z, Yu R, Liu F, Ma Q, Zhao S, Hu H, Cheng Y, Yang C, Li Z, Wang X, Hao S, Chen Y, Liu M. Recent progress on the synthesis and oxygen reduction applications of Fe-based single-atom and double-atom catalysts. J Mater Chem A. 2021;9(35):19489–507. doi:10.1039/D1TA02769G
Deng Y, Luo J, Chi B, Tang H, Li J, Qiao X, Shen Y, Yang Y, Jia C, Rao P, Liao S, Tian X. Advanced atomically dispersed metal–nitrogen–carbon catalysts toward cathodic oxygen reduction in PEM fuel cells. Adv Energy Mater. 2021;11(37):2101222. doi:10.1002/aenm.202101222
Liu F, Shi L, Lin X, Zhang B, Long Y, Ye F, Yan R, Cheng R, Hu C, Liu D, Qiu J, Dai L. Fe/Co dual metal catalysts modulated by S-ligands for efficient acidic oxygen reduction in PEMFC. Sci Adv. 2023;9(23):eadg0366. doi:10.1126/sciadv.adg0366
Xu J, Lai S, Qi D, Hu M, Peng X, Liu Y, Liu W, Hu G, Xu H, Li F, Li C, He J, Zhuo L, Sun J, Qiu Y, Zhang S, Luo J, Liu X. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021;14:1374–81. doi:10.1007/s12274-020-3186-x
Minzanova ST, Mironov VF, Arkhipova DM, Khabibullina AV, Mironova LG, Zakirova YM, Milyukov VA. Biological activity and pharmacological application of pectic polysaccharides: A review. Polym. 2018;10(12):1407. doi:10.3390/polym10121407
Noreen A, Nazli ZH, Akram J, Rasul I, Mansha A, Yaqoob N, Iqbal R, Tabasum S, Zuber M, Zia KM. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int J Biol Macromol. 2017;101:254–72. doi:10.1016/j.ijbiomac.2017.03.029
Freitas CMP, Coimbra JSR, Souza VGL, Sousa RCS. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coat. 2021;11(8):922. doi:10.3390/coatings11080922
Balakrishnan A, Chinthala M, Kumar A, Rtimi S. Construction of dual Z-scheme ternary carbon nitride homojunction pectin microspheres as a multifunctional photocatalyst for tetracycline degradation, H2O2 production, and N2 fixation. Chem Eng J. 2024;496:153899. doi:10.1016/j.cej.2024.153899
Badiei M, Asim N, Mohammad M, Akhtaruzzaman M, Samsudin NA, Amin N, Sopian K. Nanostructured polysaccharide-based materials obtained from renewable resources and uses. Innovation in Nano-Polysaccharides for Eco-sustainability. 2022;163–200. doi:10.1016/B978-0-12-823439-6.00015-5
Minzanova ST, Mironov VF, Vyshtakalyuk AB, Tsepaeva OV, Mironova LG, Smolentsev AV, Mindubaev AZ, Zobov VV, authors; Institution Russian Academy of Sciences A.E. Arbuzov Institute of Organic and Physical Chemistry of the Kazan Scientific Center of the Russian Academy of Sciences, assignee. Production line of pectin metallocomplexes. Russian Federation patent RU 107525. 2011 Aug 20.
Yarkaeva YuA, Dubrovsky DI, Zil’berg RA, Maistrenko VN, Kornilov VM. A voltammetric sensor based on a 3, 4, 9, 10-perylenetetracarboxylic acid composite for the recognition and determination of tyrosine enantiomers. J Anal Chem. 2020;75:1537–45. doi:10.1134/S1061934820110143
Laviron EJJ. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem. 1979;101(1):19–28. doi:10.1016/S0022-0728(79)80075-3
DOI: https://doi.org/10.15826/chimtech.2025.12.1.10
Copyright (c) 2024 Elgina Lebedeva, Guliya Nizameeva, Irek Nizameev, Viktoria Kuznetsova, Marsil Kadirov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







