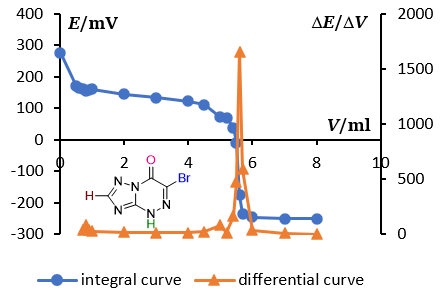
Determination of pKa of triazolo[5,1-c][1,2,4]triazines in non-aqueous media by potentiometric titration
Abstract
Keywords
Full Text:
PDFReferences
Grygorenko OO, Volochnyuk DM, Ryabukhin SV, Judd DB. The symbiotic relationship between drug discovery and organic chemistry. Chem Eur J. 2019;26(6):1196–1237. doi:10.1002/chem.201903232
Rusinov VL, Ulomskii EN, Chupakhin ON, Charushin VN. Azolo[5,1-c]-1,2,4-triazines as a new class of antiviral compounds. Russ Chem Bull. 2008;57:985–1014.
Kato R, Oshima T, Takanaka A. Studies on the mechanism of nitro reduction by rat liver. Mol Pharmacol. 1969;5(5):487–498.
Mason RP, Holtzman JL. Mechanism of microsomal and mitochondrial nitroreductase. Electron spin resonance evidence for nitroaromatic free radical intermediates. Biochem. 1975;14(8):1626–1632. doi:10.1021/bi00679a013
Kedderis GL, Miwa GT. The metabolic activation of nitroheterocyclictherapeuticagents. Drug Metab Rev. 1988;19(1):33–62. doi:10.3109/03602538809049618
Viodé C, Bettache N, Cenas N, Krauth-Siegel R L, Chauvière G, Bakalara N, Périé J. Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol. 1999;57(5):549–557. doi:10.1016/S0006-2952(98)00324-4
Wardman P. Application of pulse radiolysis methods to studythereactions and structure of biomolecules. Rep Prog Phys. 1978;41(2):259–271. doi:10.1088/0034-4885/41/2/00
Baizer MM, Lund H. Organic electrochemistry: An introduction and a guide. M. Dekker: New York;1983. 1072 p.
Gyenes I. Titration in non-aqueous media. Van Nostrand: Budapest;1967. 461 p.
Walter H. Titrations in nonaqueous solvents. Elsevier: New York and London;2012. 251 p.
Ivoilova A, Mikhalchenko LV, Tsmokalyuk A, Leonova M, Lalov A, Mozharovskaia P, Kozitsina AN, Ivanova AV, Rusinov VL. Redox conversions of 5-methyl-6-nitro-7-oxo-4,7-dihydro-1,2,4triazolo[1,5-a]pyrimidinide l-arginine monohydrate as a promising antiviral drug. Mol. 2021;26(16):5087.
Ivoilova AV, Mikhal’chenko LV, Tsmokalyuk AN, Kozitsina AN, Ivanova AV, Rusinov VL. Redox conversions of new antiviral drug Triazavirin®: electrochemical study and ESR spectroscopy. Russ Chem Bull. 2021;70(6):1099–1108. doi:10.1007/s11172-021-3190-7
Mozharovskaia PN, Ivoilova AV, RA Drokin, AV Ivanova, AN Kozitsina, VL Rusinov. The electrochemical behavior’s character of a potential antiviral drug 3-nitro-4-hydroxy-7-methylthio-4h-[1,2,4]triazolo[5,1-c][1,2,4]triazinide monohydrate. Chim Techno Acta. 2022;9(4):20229426. doi:10.15826/chimtech.2022.9.4.26
Egorova LG, Petrov AY, Rusinov VL. NH acidities of 7-oxo-4, 7-dihydropyrazolo-and 1, 2, 4-triazolo [5, 1-c][1, 2, 4] triazines. Chem of Heterocycl Compd. 1984;20:564–566.
Schmidt R, Lang F, Heckmann M. Human Physiology with Basic Pathophysiology. Volumes 1 and 2 (set). Springer-Verlag: New York;2011. 494 p.
Bersneva EV, Voinkov EK, Drokin RA, Gerasimova NA, Evstigneeva NP, Zilberberg NV, Kungurov NV, Rusinov VL. Synthesis and study of antimicrobial activity of 4-amino-1,2,4-triazolo[5,1-c][1,2,4]triazine derivatives against Neisseria gonorrhoeae. Russ Chem Bull. 2023;72:3013–3021. doi:10.1007/s11172-023-4113-6
Chupakhin ON, Rusinov VL, Varaksin MV, Ulomskiy EN, Savateev RV, Butorin II, Weijie D, Zhiyong S, Charushin VN. Triazavirin – A novel effective antiviral drug. Int J Mol Sci. 2022;23:14537. doi:10.3390/ijms232314537
Drokin RA, Fesenko EA, Mozharovskaia PN, Medvedeva MV, Svalova TS, Kozitsina AN, Esaulkova YaL, Volobueva AS, Zarubaev VV, Rusinov VL. Synthesis and antiviral activity of 6-nitro-7-oxo-4,7-dihydroazolo-[5,1-c] [1,2,4]-triazines. Russ Chem Bull. 2022;71:2460–2466. doi:10.1007/s11172-022-3674-0
Mozharovskaia PN, Ivoilova AV, Malakhova NA, Drokin RA, Balin IA, Kozitsina AN, Ivanova AV, Rusinov VL. Voltammetric Determination of a Potential Antiviral Drug Sodium Salt of 3-Nitro-4-Hydroxy-7-Methylthio-4H-[1,2,4]Triazolo[5,1-c][1,2,4]Triazinide Monohydrate. J Anal Chem. 2024;79:733–739. doi:10.1134/S1061934824700114
Rusinov VL, Ulomsky EN, Chupakhin ON, Petrov AYu, Sharonov EL. Kharakteristichnyye оsobennosti nukleofil'nogo zameshcheniya nitrogruppy v digidroazolo[5,1-c][1,2,4]-triazinakh [Characteristic Features of Nucleophilic Substitution of the Nitro Group in Dihydroazolo[5,1-c][1,2,4]-Triazines]. Chem Heterocycl Compd. 1989;2:253–257.
Rusinov VL, Ulomskii EN, Chupakhin ON, Zubairov MM, Kapustin AB, Mitin NI, Zhiravetskii MI, Vinograd IA. Synthesis and antiviral activity of 6-nitro-7-oxo-4,7-dihydroazolo-[5,1-c] [1,2,4]-triazines. Pharmaceutical Chem J. 1990;24:646–650. doi:10.1007/BF00767030
Rusinov VL, Drokin RA, Chupakhin ON, Charushin VN, Savateev KV, Ulomskij EN, Zarubaev VV, Volobueva AS, Lantseva KS. 3-nitro-4-hydroxy-7-propargylthio-[1,2,4]triazolo[5,1c][1,2,4]triazine and 3-nitro-4-hydroxy-7-ethylthio-[1,2,4]triazolo[5,1c][1,2,4]triazine with antiviral activity. Russian Federation patent RU 2775551. 2022 Jun 4.
Ivanov PM, Pojarlieff IG, Simova SD, Velinov GD. Interaction between charged groups. pK-values and conformations of the diastereomers of 3-amino-2, 3-diphenylpropanoic acids and their ester and N-acetyl derivatives. Bulgar Chem Commun. 2014;46:9–15.
Neese F. The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci. 2012;2(1):73–78. doi:10.1002/wcms.81
Neese F. Software update: the ORCA program system, version 4.0. Wiley Interdiscip Rev. Comput Mol Sci. 2017;8:e1327. doi:10.1002/wcms.1327
Neese F, Wennmohs F, Becker U, Riplinger C. The ORCA quantum chemistry program package. J Chem Phys. 2020;152(22):224108. doi:10.1063/5.0004608
Najibi A, Goerigk L. The nonlocal kernel in van der Waals density functionals as an additive correction: An extensive analysis with special emphasis on the B97M-V and ωB97M-V approaches. J Chem Theory Comput. 2018;14(11):5725–5738. doi:10.1021/acs.jctc.8b00842
Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys. 2005;7(18):3297–3305. doi:10.1039/B508541A
Weigend F. Accurate Coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys. 2006;8(9):1057–1065. doi:10.1039/B515623H
York DM, Karplus M. A smooth solvation potential based on the conductor-like screening model. J Phys Chem A. 1999;103(50):11060–11079. doi:10.1021/jp992097l
Tian L, Chen F. Multiwfn: A Multifunctional Wavefunction Analyzer. J Comput Chem. 2012;33(5):580–592. doi:10.1002/jcc.22885
Dennington R, Keith T, Millam J. GaussView, Version 6.1.1. Semichem Inc. Shawnee Mission:Kansas; 2019.
Gordon AJ, Ford RA. The chemist's companion: a handbook of practical data, techniques, and references. John Wiley & Sons:Canada; 1973. 560 p.
Gurtovenko A A., Anwar J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J Phys Chem. B 2007;111(35):10453–10460.
Amatore C, Capobianco G, Farnia G, Sandona G, Saveant JM, Severin MG, Vianello E. Kinetics and mechanism of self-protonation reactions in organic electrochemical processes. J Am Chem Soc. 1985;107(7):1815–1824. doi:10.1021/ja00293a003
Farnia G, Mengoli G, Vianello E. Electrode reaction mechanism of nitroderivatives inaprotic solvents: Part 1. m-nitrophenol. J Electroanal Chem. 1974;50(1):73–89. doi:10.1016/S0022-0728(74)80278-0
Po HN, Senozan NM. The Henderson-Hasselbalch equation: its history and limitations. J Chem Educ. 2001;78(11):1499. doi:10.1021/ed078p1499
Artemyev GA. Triazavirin – protivovirusnyy preparat novogo pokoleniya [Triazavirin is a new generation antiviral drug]. Yekaterinburg:Russia; 2016. 254 p.
D Augustin-Nowacka, Makowski M, Chmurzynski L. Acid–base equilibria in systems involving substituted pyridines in polar aprotic protophobic media and in the amphiprotic methanol. Anal Chim Acta. 2000;418(2):233–240. doi:10.1016/S0003-2670(00)00967-3
Zuman P. Half a century of research using polarography. Microchem J. 1997;57(1):4–51. doi:10.1006/mchj.1997.1468
Huie WR. Nitrone spin traps for biological studies (Volumes I and II): Doctoral dissertation of chemistry [dissertation]. Louisiana State University and Agricultural & Mechanical College; 1987. 438 p. doi:10.31390/gradschool_disstheses.4451
DOI: https://doi.org/10.15826/chimtech.2024.11.4.10
Copyright (c) 2024 Alexandra Vs. Ivoilova, Polina N. Mozharovskaia, Liya V. Mokrousova, Ivan A. Balin, Anton N. Tsmokalyuk, Roman A. Drokin, Irina M. Sapozhnikova, Vladimir L. Rusinov, Alla Vl. Ivanova, Alisa N. Kozitsina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







