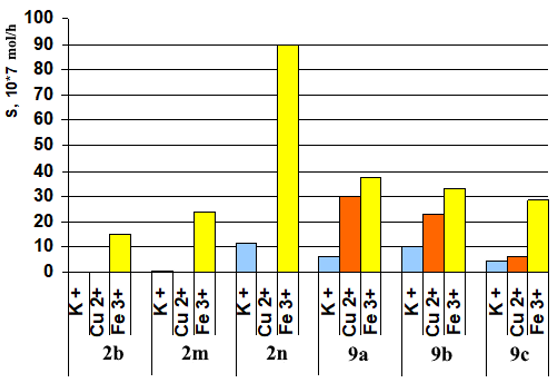
Antituberculosis activity of some 3,6-disubstituted 1,2,4,5-tetrazines
Abstract
Keywords
Full Text:
PDFReferences
Bhardwaj P, Gupta N. 1,2,4,5-Tetrazines as platform molecules for energetic materials and pharmaceuticals. Iran J. Org. Chem. 2016;8:1827–1831.
Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Three components coupling catalysed by NaHSO4∙SiO2 – a convenient synthesis, antibacterial and antifungal activities of novel 6- Aryl-1,2,4,5-tetrazinan-3-ones. J Enzym Inhib Med Chem. 2008;23:87–93. doi:10.1080/14756360701421351
Nhu D, Duffy S, Avery VM, Hughes A, Baell JB. Antimalarial 3-arylamino-6-benzylamino-1,2,4,5-tetrazines. Bioorg Med Chem Lett. 2010;20(15):4496–8. doi:10.1016/j.bmcl.2010.06.036
Ishmetova RI, Ignatenko NK, Belyaninova IA, Gerasimova NA, Evstigneeva NP, Zilberberg NV, Kungurov NV, Rusinov GL, Charushin VN, Chupakhin ON, inventors; I. Postovsky Institute of Organic Synthesis, UD RAS, assignee. Selective Antibacterial agents, represented by 3-(azol-1-yl)-6-amino-substituted 1,2,4,5-tetrazines. Russian Federation patent RU 2642882. 2018 Jan 30. Russian.
Ishmetova RI, Babkov DA, Kucheryavenko AF, Babkova VS, Sirotenko VS, Ignatenko NK, Tolschina SG, Vassiliev PM, Rusinov GL, Spasov AA. In silico consensus activity prediction, rational synthesis, and evaluation of antiglycation and antiplatelet activities of 3,6-disubstituted 1,2,4,5-tetrazines. Russ Chem Bull. 2020;69:768–73. doi:10.1007/s11172-020-2831-6
Rao G-W, Hu W-X. Synthesis, structure analysis, and antitumor activity of 3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg Med Chem Lett. 2006;16:3702–3705. doi:10.1016/j.bmcl.2006.04.066
Can˜ete-Molina A, Espinosa-Bustos C, Gonza´lez-Castro M, Fau´ndez M, Mella J, Tapia RA, Cabrera AR, Brito I, Aguirre A, Salas CO. Design, synthesis, cytotoxicity and 3D-QSAR analysis of new 3,6-disubstituted-1,2,4,5-tetrazine derivatives as potential antitumor agents. Arab J Chem. 2019;12:1092–1107. doi:10.1016/j.arabjc.2017.04.002
Hazhazi H, Melkemi N, Salah T, Bouachrine M. DFT-based reactivity and combined QSAR, molecular docking of 1,2,4,5-Tetrazine derivatives as inhibitors of Pim-1 kinase. Heliyon. 2019;5:02451. doi:10.1016/j.heliyon.2019.e02451
Rusinov GL, Latosh NI, Ishmetova RI, Kravchenko MA, Ganebnykh IN, Sokolov VA, Chupakhin ON. Synthesis and Tuberculostatic Activity of Some Substituted Amino Acid Methyl Esters with Sym-Tetrazine Moieties. Pharm Chem J. 2005;39:8–10. doi:10.1007/s11094-005-0068-1
Ishmetova RI, Ganebnykh IN, Ignatenko NK, Tolshchina SG, Korotina AV, Eltsov OS, Kravchenko MA, Rusinov GL. Synthesis and tuberculostatic activity of new 3-alkylthio-6-R-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazines. Russ Chem Bull. 2021;70:1093–8. doi:10.1007/s11172-021-3189-0
Ishmetova RI, Ganebnykh NK, Ignatenko IN, Tolshchina SG, Korotina AV, Kravchenko MA, Skornyakov SN, Rusinov GL. Synthesis and tuberculostatic activity of new 3-alkylthio-6-R-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazines. Russ. Chem. Bull. 2014;63:1423–30. doi:10.1007/s11172-014-0613-8
Bekker OB, Danilenko VN, Ishmetova RI, Korotina AV, Maslov DA, Rusinov GL, Tolshchina SG, Charushin VN, inventors; I. Postovsky Institute of Organic Synthesis, UD RAS, assignee. Antituberculous therapeutic agent: composition of imidazo[1,2-b]tetrazine and pyrazinamide. Russian Federation patent RU 2545458. 2015 Mar 27. Russian.
Salari N, Kanjoori AN, Hosseinian-Far A, Hasheminezhad R, Mansouri K, Mohammadi M. Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis. Infect. Dis Poverty. 2023;12–57. doi:10.1186/s40249-023-01107-x
Coburn MD, Buntain GA, Harris BW, Hiskey MA, Lee KY, Ott DG. An improved synthesis of 3,6-diamino-1,2,4,5-tetrazine. II. From triaminoguanidine and 2,4-pentanedione. J Heterocycl Chem. 1991;28:2049–50. doi:10.1002/jhet.5570280844
Rusinov GL, Latosh NI, Ganebnykh IN, Ishmetova RI, Ignatenko NК, Chupakhin ON. Synthesis of 1,2,4,5-tetrazines, symmetrically and unsymmetrically 3,6-disubstituted by N-nucleophiles. Russ J Org Chem. 2006;42:757–65. doi:10.1134/S1070428006050198
Latosh NI, Rusinov GL, Ganebnykh IN, Chupakhin ON, Pyrazole as a leaving group in nucleophilic substitution in 3,6-bis(3,5-dimethyl-1-pyrazolyl)-1, 2, 4, 5-tetrazines. Russ J Org Chem. 1999;35:1363–71.
Tolshchina SG, Vyakhireva AG, Ignatenko NK, Ishmetova R I, Ganebnykh IN, Slepukhin PA, Rusinov GL. Cyclization of (1,2,4,5-tetrazin-3-yl)hydrazones to 3,7-dihydro-1,2,4-triazolo[4,3-b]-1,2,4,5-tetrazines. Russ Chem Bull. 2009;58:1281–90. doi:10.1007/s11172-009-0168-2
Rusinov GL, Ishmetova RI, Ganebnykh IN, and Chupakhin ON. Hetaryl displacement in 3, 6-disubstituted 1, 2, 4, 5-tetrazines with anhydro bases of N-methylquinaldiniums. Heterocycl Commun. 2006;12:99–102. doi:10.1515/HC.2006.12.2.99
Tolshchina SG, Ishmetova RI, Ignatenko NK, Korotina AV, Slepukhin PA, Rusinov GL, Charushin VN. Synthesis and transformations of cyanomethyl-1,2,4,5-tetrazines. J Heterocycl Chem. 2013;49:604–17. doi:10.1007/s10593-013-1288-z
Ganebnykh IN. Synthesis and transformations of 3,6-disubstituted and azoloannelated s-tetrazines [dissertation of candidate of chemical sciences]. Ekaterinburg (Russia): I. Ya. Postovsky RAS; 2003. 229 p.
Kudon S, Kudon T. Study on the Isolation Culture Technigueof Tubercle Bacilli Applicatible in Remote Areas, Part 1, Kekkaku (Tuberculosis). PMID: 4205280. Japanese. 1973;48(10):453–62.
Kobuke Y, Katsumi H, Hanji K, Horiguchi K. Macrocyclic ligands composed of tetrahydrofuran for selective transport of monovalent cations through liquid membrane. J Am Chem Soc. 1976;98:7414–9. doi:10.1021/ja00439a050
Shey-Njila O, Hikal AF, Gupta T, Sakamoto K, Azami HY, Watford WT, Quinn FD, Karls RK. CtpB Facilitates Mycobacterium Tuberculosis Growth in Copper-Limited Niches. Int J Mol Sci. 2022;23:5713. doi:10.3390/ijms23105713
DOI: https://doi.org/10.15826/chimtech.2024.11.4.14
Copyright (c) 2024 Rashida I. Ishmetova, Ilya N. Ganebnykh, Nina K. Ignatenko, Irina G. Ovchinnikova, Olga V. Fedorova, Sergei N. Skornyakov, Gennady L. Rusinov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







