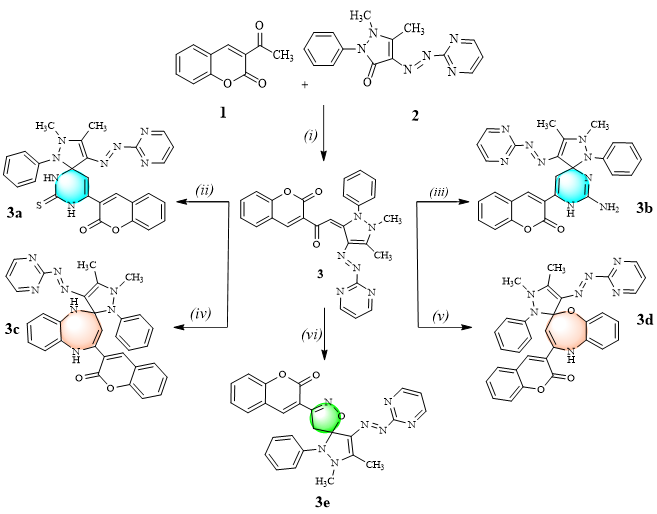
New spiro-heterocyclic coumarin derivatives as antibacterial agents: design, synthesis and molecular docking
Abstract
Keywords
Full Text:
PDFReferences
Rodriguez-Enriquez F, Costas-Lago C, Besada P, Alonso-Pena M, Torres-Teran I, Vina D, Teran C. Novel coumarin-pyridazine hybrids as selective MAO-B inhibitors for the Parkinson’s disease therapy. Bioorg Chem. 2020;104:104203. doi:10.1016/j.bioorg.2020.104203
García-Beltrán O, Mena NP, Aguirre P, Barriga-Gonzalez G, Galdámez A, Nagles E, Núñez MT. Development of an iron-selective antioxidant probe with protective effects on neuronal function. J PloS one. 2017;12(12):0189043. doi:10.1371/journal.pone.0189043
Aguirre P, García-Beltrán O, Tapia V, Muñoz Y, Cassels BK, Núñez MT. Neuroprotective effect of a new 7, 8-dihydroxycoumarin-based Fe2+/Cu2+ chelator in cell and animal models of Parkinson’s disease. ACS Chem Neurosci. 2017;8(1):178-185. doi:10.1021/acschemneuro.6b00309
Al-Abbasy OY, Younus SA, Rashan AI, Ahmad OA. Maillard reaction: formation, advantage, disadvantage and control. A review. Food Sci Appl Biotech. 2024;7(1):145–161. doi:10.30721/fsab2024.v7.i1.333
Shen YF, Liu L, Feng CZ, Hu Y, Chen C, Wang GX, Zhu B. Synthesis and antiviral activity of a new coumarin derivative against spring viremia of carp virus. Fish Shellfish Immunol. 2018;81:57-66. doi:10.1016/j.fsi.2018.07.005
Sahoo CR, Paidesetty SK, Sarathbabu S, Dehury B, Senthil Kumar N, Padhy RN. Molecular dynamics simulation, synthesis and topoisomerase inhibitory actions of vanillin derivatives: a systematic computational structural integument. J Biomol Struct Dyn. 2022;40(22):11653-11663. doi:10.1080/07391102.2021.1961867
Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, Gallelli L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human microRNA modulation. Molecules. 2019;25(1):63. doi:10.3390/molecules25010063
Ali OT, Mohammed MO, Al Dulayymi JR, Baird MS. Synthesis of a di-mycoloyl tri-arabinofuranosyl glycerol fragment of the mycobacterial cell wall, based on synthetic mycolic acids. Molecules. 2019;24(19):3596. doi:10.3390/molecules24193596
Galvin CJ, Hobson M, Meng JX, Ierokomos A, Ivanov IE, Berger JM, Bryant Z. Single-molecule dynamics of DNA gyrase in evolutionarily distant bacteria Mycobacterium tuberculosis and Escherichia coli. Bio chem; 2023: 299(5). doi:10.1016/j.jbc.2023.103003
Khan T, Sankhe K, Suvarna V, Sherje A, Patel K, Dravyakar BDN. A gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed Pharmacother; 2018; 103: 923-938. doi:10.1016/j.biopha.2018.04.021
Maxwell A. DNA gyrase as a drug target. Trends Microbiol. 1997;5:102–109. doi:10.1016/S0966-842X(96)10085-8
Rashan AI, Altaee RT, Salh FS, AL-Abbasy OY, Al-Lehebe N. The role of polyamines in plants: A review. Plant Sci Today. 2023;10:164–171. doi:10.14719/pst.2520
Patil SA, Unki SN, Kulkarni AD, Naik VH, Badami PS. Co (II), Ni (II) and Cu (II) complexes with coumarin-8-yl Schiff-bases: spectroscopic, in vitro antimicrobial, DNA cleavage and fluorescence studies. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79(5):1128-1136. doi:10.1016/j.saa.2011.04.032
Abdelmohsen SA, El-Emary T. Synthesis, characterization and antimicrobial activity of novel pyrazolo [3, 4-b] pyridines and their spiro-hetero cyclic derivatives. J Adv Chem. 2014;10(7). doi:10.24297/jac.v10i7.6802
Patagar D, Uttarkar A, Patra SM, Patil JH, Kusanur R, Niranjan V, Kumar HA. Spiro benzodiazepine substituted fluorocoumarins as potent anti-anxiety agents. Russ J Bioorg Chem. 2021;47(2):390-398. doi:10.1134/S1068162021020199
Abd Allah OA, Elkhayat ES, Moustafa AH, Fahmy AA. Synthesis and Characterization of Some Novel Indole-2-One-Coumarin Hybrids. Sohag J Sci. 2024;9(2):186-189. doi:10.21608/sjsci.2024.248555.1148
Özer H, Sökmen M, Güllüce M, Adigüzel A, Şahin F, Sökmen A, Bariş Ö. Chemical composition and antimicrobial and antioxidant activities of the essential oil and methanol extract of Hippomarathrum microcarpum (Bieb.) from Turkey. J Agric Food. 2007;55(3):937-942. doi:10.1021/jf0624244
Vashistha VK, Mittal A, Bala R, DASa DK, Singh PP. Synthesis, characterization, electrochemical and antibacterial studies of mn4-type macrocyclic complexes of Ni (II). Rev Roum Chim. 2023;68(9):447-452. doi:10.33224/rrch.2023.68.9.05
Almaghrabi M, Musa A, Aljohani AK, Ahmed HE, Alsulaimany M, Miski SF, El-Agrody AM. Introducing of novel class of pyrano [2, 3-c] pyrazole-5-carbonitrile analogs with potent antimicrobial activity, DNA gyrase inhibition, and prominent pharmacokinetic and CNS toxicity profiles supported by molecular dynamic simulation. ACS Omega. 2023;8(20):17948–17965. doi:10.1080/07391102.2023.2252088
Rodriguez SV, Guíñez RF, Matos MJ, Azar CO, Maya JD, Santana L, Borges F. Synthesis and trypanocidal properties of new coumarin-chalcone derivatives. Med Chem. 2015;(5):173-177. doi:10.4172/2161-0444.1000260
Ahmed EY, Abdelhafez OM, Zaafar D, Serry AM, Ahmed YH, El‐Telbany RFA, Ali HI. Antitumor and multikinase inhibition activities of some synthesized coumarin and benzofuran derivatives. Archiv der Pharmazie. 2022;355(6):2100327. doi:10.1002/ardp.202100327
Dar PA, Bhat BA, Mir MA, Chaudhari SY, Shah WA. Synthesis, biological profile and computational insights of new derivatives of benzo [B][1, 4] diazepines as prospective anticancer agents for inhibiting the CDK-2 protein. J Biomol Struct Dyn. 2024:1-16. doi:10.1080/07391102.2024.2314270
Solankee A, Tailor R. Synthesis, characterization and biological screening of s-triazine based chalcones and its derivatization into phenyl pyrazolines, isoxazoles. Int Lett Chem Phys Astron. 2015;47:109-119. doi:10.56431/p-jy3j82
DOI: https://doi.org/10.15826/chimtech.2024.11.3.08
Copyright (c) 2024 Abdallah F. Al-burgus, Omar T. Ali, Omar Y. Al-abbasy

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







