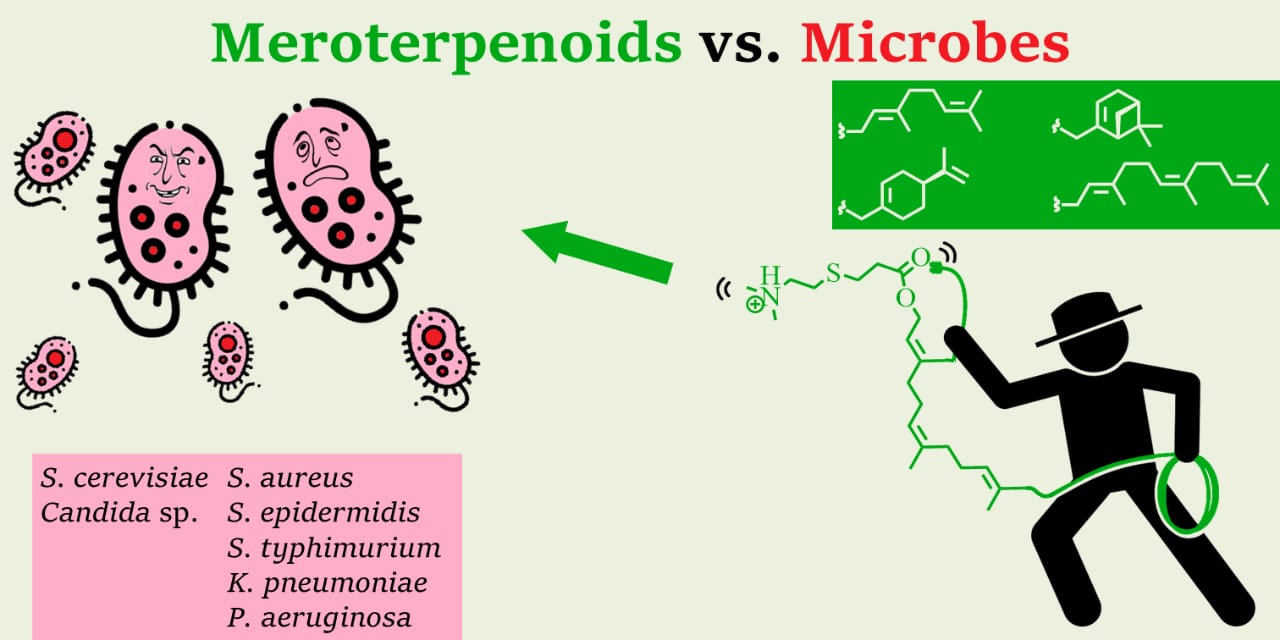
Cationic amphiphilic meroterpenoids: synthesis, antibacterial, antifungal and mutagenic activity
Abstract
Keywords
Full Text:
PDFReferences
Fair RJ, Tor Y. Antibiotics and bacterial Resistance in the 21st Century Perspect. Med Chem. 2014;6:14459. doi:10.4137/PMC.S14459
Arastehfar A, Gabaldón T, Garcia-Rubio R, Jenks JD, Hoenigl M, Salzer HJF, Ilkit M, Lass-Flörl C, Perlin DS. Drug-resistant Fungi: an Emerging challenge Threatening our Limited antifungal. Armamentarium Antibiotics. 2020;9(12):877. doi:10.3390/antibiotics9120877
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Aboderin AO, Al-Abri SS, Awang Jalil N, Benzonana N, Bhattacharya S, Brink AJ, Burkert FR, Cars O, Cornaglia G, Dyar OJ, Friedrich AW, Gales AC, Gandra S, Giske CG, Goff DA, Goossens H, Gottlieb T, Guzman Blanco M, Hryniewicz W, Kattula D, Jinks T, Kanj SS, Kerr L, Kieny MP, Kim YS, Kozlov RS, Labarca J, Laxminarayan R, Leder K, Leibovici L, Levy-Hara G, Littman J, Malhotra-Kumar S, Manchanda V, Moja L, Ndoye B, Pan A, Paterson DL, Paul M, Qiu H, Ramon-Pardo P, Rodríguez-Baño J, Sanguinetti M, Sengupta S, Sharland M, Si-Mehand M, Silver LL, Song W, Steinbakk M, Thomsen J, Thwaites GE, van der Meer JWM, Van Kinh N, Vega S, Villegas MV, Wechsler-Fördös A, Wertheim HFL, Wesangula E, Woodford N, Yilmaz FO, Zorzet A. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022. Available from: https://www.who.int/publications/i/item/9789240060241, Accessed on 26 April 2024.
Sant DG, Tupe SG, Ramana CV, Deshpande MV. Fungal cell membrane-promising drug target for antifungal therapy. J Appl Microbiol. 2016;121(6):1498–1510. doi:10.1111/jam.13301
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope cold spring. Harb Perspect Biol. 2010;2(5):a000414–a000414. doi:10.1101/cshperspect.a000414
Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem. 2010;11(1):35–45. doi:10.1002/cbic.200900557
Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet. 2013;33–82. doi:10.1016/B978-0-12-407677-8.00002-6
Virtanen JA, Cheng KH, Somerharju P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc National Acad Sci. 1998;95(9):4964–4969. doi:10.1073/pnas.95.9.4964
Cook MA, Wright GD The past, present, and future of antibiotics Sci. Transl. Medicine 2022; 14(657): eabo7793 doi:10.1126/scitranslmed.abo7793
Wall G, Lopez-Ribot JL. Current antimycotics, new prospects, and future approaches to antifungal. Therapy Antibiotics. 2020;9(8):445. doi:10.3390/antibiotics9080445
Herzog IM, Fridman M. Design and synthesis of membrane-targeting antibiotics: from peptides- to aminosugar-based antimicrobial cationic amphiphiles MedChemComm. 2014;5(8):1014–1026. doi:10.1039/C4MD00012A
Obłąk E, Piecuch A, Rewak-Soroczyńska J, Paluch E. Activity of gemini quaternary ammonium salts against microorganisms. Appl Microbiol Biotechnol. 2019;103(2):625–632. doi:10.1007/s00253-018-9523-2
Ahmady AR, Hosseinzadeh P, Solouk A, Akbari S, Szulc AM, Brycki BE. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv Colloid Interface Sci. 2022;299:102581. doi:10.1016/j.cis.2021.102581
Olsufyeva EN, Yankovskaya VS. Main trends in the design of semi-synthetic antibiotics of a new generation. Russ Chem Rev. 2020;89(3):339–378. doi:10.1070/RCR4892
Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86–96. doi:10.1016/j.bcp.2016.11.019
Obydennov KL, Kalinina TA, Ryabova DV, Kosterina MF, Glukhareva TV. 2-(4-Oxo-1,3-thiazolidin-2-ylidene)acetamid as promising scaffold for designing new antifungal compounds. Chim Techno Acta. 2023;10(1):202310106. doi:10.15826/chimtech.2023.10.1.06
Megha GV, Bodke YD, Shanavaz H, Joy MN. Substituted benzocoumarin derivatives: synthesis, characterization, biological activities and molecular docking with ADME studies. Chim. Techno Acta. 2022;9(4):20229419. doi:10.15826/chimtech.2022.9.4.19
Shurpik DN, Aleksandrova YI, Makhmutova LI, Akhmedov AA, Stoikov II. Towards nanomaterials with tubular pores: synthesis and self-assembly of bis-pillar[5]arene. Chim Techno Acta. 2023;10(4):202310412. doi:10.15826/chimtech.2023.10.4.12
Shurpik DN, Akhmedov AA, Cragg PJ, Plemenkov VV, Stoikov II. Progress in the chemistry of macrocyclic Meroterpenoids Plants. 2020;9(11):1582. doi:10.3390/plants9111582
Plemenkov VV, Shurpik DN, Akhmedov AA, Puplampu JB, Stoikov II. Progress in studies on meroterpenoids. Bioact Nat Prod. 2020;64:181–216. doi:10.1016/B978-0-12-817903-1.00006-1
Amirzakariya BZ, Shakeri A Bioactive terpenoids derived from plant endophytic fungi: an updated review (2011–2020). Phytochem. 2022;197:113130. doi:10.1016/j.phytochem.2022.113130
Akhmedov AA, Panina YV, Gamirov RR, Shurpik DN, Stoikov II Synthesis of perillyl-containing meroterpenoids and their supramolecular self-assembly with pillar[5]arene. Russ Chem Bull. 2024;73(3):644–652. doi:10.1007/s11172-024-4174-1
Akhmedov AA, Shurpik DN, Padnya PL, Khadieva AI, Gamirov RR, Panina YV, Gazizova AF, Grishaev DY, Plemenkov VV, Stoikov II. Supramolecular amphiphiles based on pillar[5]arene and meroterpenoids: synthesis, self-association and interaction with floxuridine. Int J Mol Sci. 2021;22(15):7950. doi:10.3390/ijms22157950
Akhmedov AA, Shurpik DN, Plemenkov VV, Stoikov II. Water-soluble meroterpenes containing an aminoglyceride fragment with geraniol residues: synthesis and membranotropic properties. Mendeleev Commun. 2019;29(1):29–31. doi:10.1016/j.mencom.2019.01.008
Zacchino SA, Butassi E, Liberto MD, Raimondi M, Postigo A, Sortino M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs Phytomed. 2017;37:27–48. doi:10.1016/j.phymed.2017.10.018
Akhmedov A, Gamirov R, Panina Y, Sokolova E, Leonteva Y, Tarasova E, Potekhina R, Fitsev I, Shurpik D, Stoikov I. Towards potential antifungal agents: synthesis, supramolecular self-assembly and in vitro activity of azole mono-, sesqui- and diterpenoids. Org Biomol Chem. 2023;21(23):4863–4873. doi:10.1039/D3OB00528C
Li-Zhulanov NS, Ponomarev KY, Sari S, Gülmez D, Arikan-Akdagli S, Krasnov VI, Suslov EV, Volcho KP, Salakhutdinov NF. Myrtenyl-bispidine containing azole: synthesis and antifungal activity. Mendeleev Commun. 2024;34(1):119–121. doi:10.1016/j.mencom.2024.01.036
Li-Zhulanov NS, Zaikova NP, Sari S, Gülmez D, Sabuncuoğlu S, Ozadali-Sari K, Arikan-Akdagli S, Nefedov AA, Rybalova TV, Volcho KP, Salakhutdinov NF. Rational design of new monoterpene-containing azoles and their antifungal activity. Antibiotics. 2023;12(5):818. doi:10.3390/antibiotics12050818
Vashishth A, Saini S, Garg VK, Abdulabbas HS, Kumar A, Sethi P, Seth P, Singh B, Tuli HS. Phytochemicals from medicinal plants as antiviral agents: recent trends and advancements. Asian J Chem. 2023;35(6):1303–1314. doi:10.14233/ajchem.2023.27927
Jahangeer M, Fatima R, Ashiq M, Basharat A, Qamar SA, Bilal M, Iqbal HMN. Therapeutic and biomedical Potentialities of terpenoids – A Review. J Pure Appl Microbiol. 2021;15(2):471–483. doi:10.22207/JPAM.15.2.04
Gonzalez-Burgos E, Gomez-Serranillos MP. Terpene compounds in Nature: A review of their Potential antioxidant Activity. Curr Med Chem. 2012;19(31):5319–5341. doi:10.2174/092986712803833335
Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321–324. doi:10.1016/j.ymeth.2007.01.006
Mortelmans K, Zeiger E. The ames Salmonella/microsome mutagenicity assay. Mutat Res Fundam Mol Mech Mutagen. 2000;455(1–2):29–60. doi:10.1016/S0027-5107(00)00064-6
Levy DD, Zeiger E, Escobar PA, Hakura A, van der Leede BM, Kato M, Moore MM, Sugiyama K. Recommended criteria for the evaluation of bacterial mutagenicity data (Ames test). Mutat Res Genet Toxicol Environ Mutagen. 2019;848:403074. doi:10.1016/j.mrgentox.2019.07.004
Rulev Yu. A Aza‐michael reaction: a decade later – is the research over? Eur J Org Chem. 2023;26(26):e202300451. doi:10.1002/ejoc.202300451
Roseli RB, Keto AB, Krenske EH. Mechanistic aspects of thiol additions to michael acceptors: insights from computations WIREs. Comput Mol Sci. 2023;13(2):e1636. doi:10.1002/wcms.1636
Mukherjee A, Chatterjee R, De A, Samanta S, Mahato S, Ghosal NC, Zyryanov GV, Majee A. Conjugated addition of amines to electron deficient alkenes: a green approach. Chim Techno Acta. 2017;4(2):140–147. doi:10.15826/chimtech/2017.4.2.029
Lopes AP, de Oliveira Castelo Branco RR, de Alcântara Oliveira FA, Campos MAS, de Carvalho Sousa B, Agostinho RC, Gonzalez AGM, Rocha JA, Pinheiro REE, Araújo AR, dos Santos Soares MJ. Antimicrobial, modulatory, and antibiofilm activity of tt-farnesol on bacterial and fungal strains of importance to human health. Bioorg Med Chem Lett. 2021;47:128192. doi:10.1016/j.bmcl.2021.128192
Bonifait L, Marquis A, Genovese S, Epifano F, Grenier D. Synthesis and antimicrobial activity of geranyloxy- and farnesyloxy-acetophenone derivatives against oral pathogens. Fitoterapia. 2012;83(6):996–999. doi:10.1016/j.fitote.2012.06.003
Zhang HZ, Damu GLV, Cai GX, Zhou CH. Design, synthesis and antimicrobial evaluation of novel benzimidazole type of fluconazole analogues and their synergistic effects with Chloromycin, norfloxacin and fluconazole. Eur J Med Chem. 2013;64:329–344. doi:10.1016/j.ejmech.2013.03.049
Cuenca-Estrella M, Díaz-Guerra TM, Mellado E, Monzón A, Rodríguez-Tudela JL. Comparative in vitro Activity of voriconazole and itraconazole Against Fluconazole-susceptible and Fluconazole-resistant Clinical isolates of candida Species from Spain. Eur J Clin Microbiol Infect Dis. 1999;18(6):432–435. doi:10.1007/s100960050313
Correa RMS, Mota TC, Guimarães AC, Bonfim LT, Burbano RR, Bahia MO. Cytotoxic and genotoxic Effects of fluconazole on african Green monkey Kidney (Vero) cell line. BioMed Res Int. 2018;1–7. doi:10.1155/2018/6271547
DOI: https://doi.org/10.15826/chimtech.2024.11.2.09
Copyright (c) 2024 Alan Akhmedov, Rustem Gamirov, Yulia Panina, Alina Baklagina, Evgenia Sokolova, Pavel Zelenikhin, Olga Babaeva, Vasily Babaev, Dmitriy Shurpik, Ivan Stoikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







