Modification of bacterial cellulose using silk fibroin β-sheet crystals induced by ultrasonication
Abstract
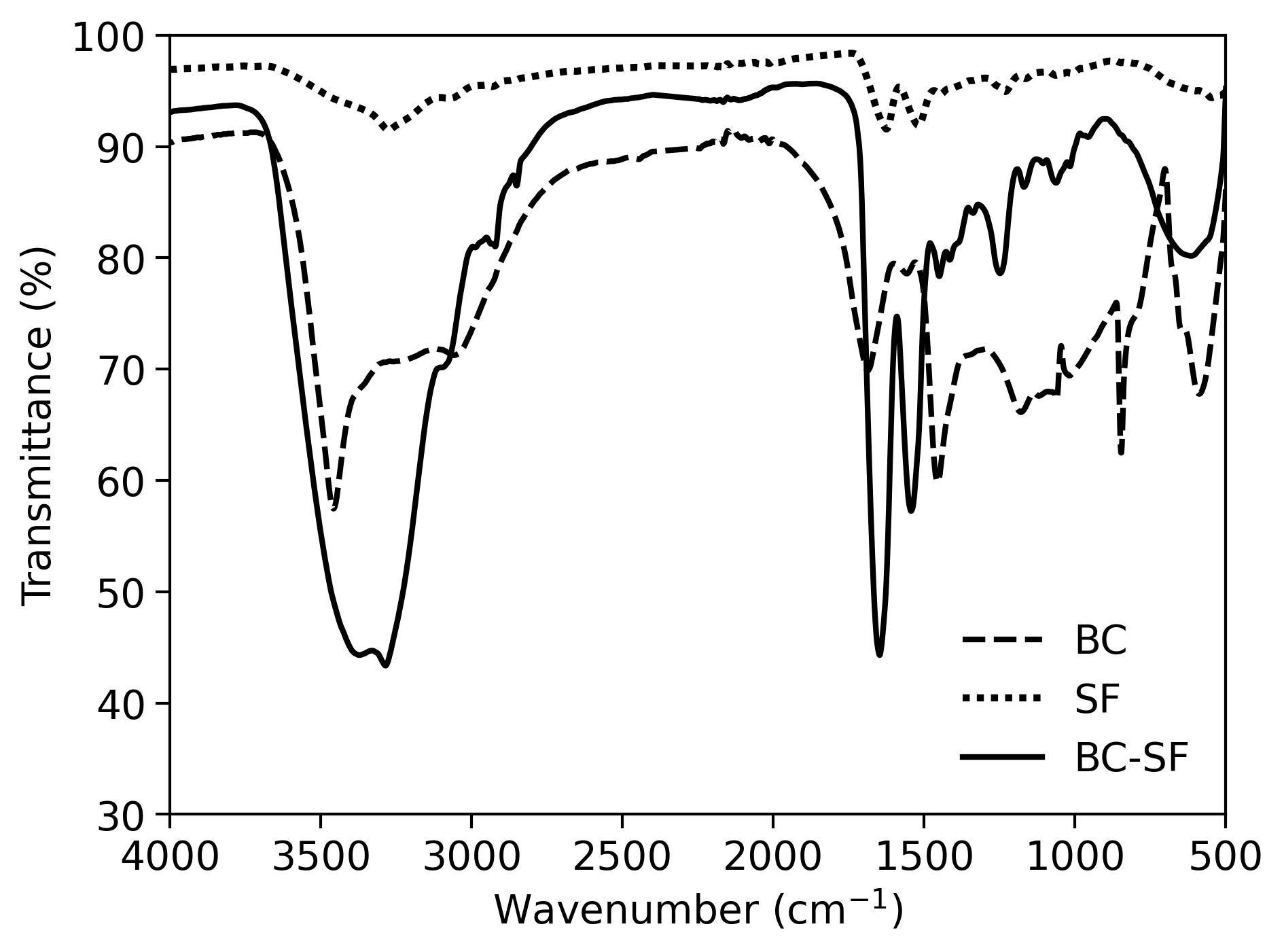
Silk fibroin (SF) has been continuously explored as a biomaterial due to its biocompatibility, tunability, and self-healing properties. In this work, we present a novel approach to the modification of bacterial cellulose (BC) with SF β-sheet dominant structures induced via ultrasonication. Secondary structure analysis through infrared spectroscopy, thioflavin T assay, and circular dichroism spectropolarimetry revealed a conversion of silk I to silk II structures within the protein mixture. Cold field emission scanning electron microscope images revealed the tightly packed fibers coated with the protein. Thermogravimetric curves demonstrated higher resistance to temperature degradation supplemented by broader and flatter DSC curves attributed to the highly bonded and dense composite. Successful conversion of amide I to amide II and amide III allowed for the more stable β-crystals to contribute to a more thermodynamically stable double-network hydrogel. The conversion of silk I to silk II structures offers a viable and highly biocompatible material that is both thermodynamically and biochemically stable for various potential biomedical applications.
Keywords
Full Text:
PDFReferences
Angelova N, Hunkeler D. Rationalizing the design of polymeric biomaterials. Trends Biotechnol. 1999;17:409–421. doi:10.1016/s0167-7799(99)01356-6
Liu J, Ge X, Liu L, Xu W, Shao R. Challenges and opportunities of silk protein hydrogels in biomedical applications. Mater Adv. 2022;3:2291–2308. doi:10.1039/D1MA00960E
Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, et al. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 2015;46:86–110. doi:10.1016/j.progpolymsci.2015.02.001
Wang C, Xia K, Zhang Y, Kaplan DL. Silk-based advanced materials for soft electronics. Acc Chem Res. 2019;52:2916–2927. doi:10.1021/acs.accounts.9b00333
Huang W, Ling S, Li C, Omenetto FG, Kaplan DL. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem Soc Rev. 2018;47:6486–6504. doi:10.1039/c8cs00187a
Hardy JG, Scheibel TR. Composite materials based on silk proteins. Prog Polym Sci. 2010;35:1093–1115. doi:10.1016/j.progpolymsci.2010.04.005
Matsumoto A, Chen J, Collette AL, Kim U-J, Altman GH. Mechanisms of silk fibroin sol−gel transitions. J Phys Chem B. 2006;110:21630–21638. doi:10.1021/jp056350v
Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, et al. Structure and properties of silk hydrogels. Biomacromolec. 2004;5:786–792. doi:10.1021/bm0345460
Choi Y, Cho SY, Heo S, Jin HJ. Enhanced mechanical properties of silk fibroin-based composite plates for fractured bone healing. Fibers Polym. 2013;14:266–270. doi:10.1007/s12221-013-0266-5
Gregory DA, Tripathi L, Fricker ATR, Asare E, Orlando I, et al. Bacterial cellulose: a smart biomaterial with diverse applications. Mater Sci Eng R Rep. 2021;145:100623. doi:10.1016/j.mser.2021.100623
Phisalaphong M, Chiaoprakobkij N. Applications and products-nata de coco in bacterial nano cellulose. CRC Press. 2016;143. doi:10.1201/b12936-8
Gallegos AMA, Herrera CS, Parra R, Keshavarz T, Iqbal HMN. Bacterial cellulose: a sustainable source to develop value-added products – a review. BioResources. 2016;11:5641–5655. doi:10.15376/biores.11.2.Gallegos
Jung HI, Jeong JH, Lee OM, Park GT, Kim KK, et al. Influence of glycerol on production and structural–physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour Technol. 2010;101:3602–3608. doi:10.1016/j.biortech.2009.12.111
Castro C, Zuluaga R, Putaux JL, Caro G, Mondragon I et al. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym. 2011;84:96–102. doi:10.1016/j.carbpol.2010.10.072
Czaja W, Romanovicz D, Brown RM. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose. 2004;11:403–411. doi:10.1023/B:CELL.0000046412.11983.61
Gea S, Reynolds CT, Roohpour N, Wirjosentono B, Soykeabkaew N et al. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour Technol. 2011;102:9105–9110. doi:10.1016/j.biortech.2011.04.077
Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12:586–610. doi:10.1111/1751-7915.13392
Lee JM. The fixation effect of a silk fibroin–bacterial cellulose composite plate in segmental defects of the zygomatic arch: an experimental study. JAMA Otolaryngol. Neck Surg. 2013;139:629–635. doi:10.1001/jamaoto.2013.3044
Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomater. 2008;29:1054–1064. doi:10.1016/j.biomaterials.2007.11.003
Chen J, Zhuang A, Shao H, Hu X, Zhang Y. Robust silk fibroin/bacterial cellulose nanoribbon composite scaffolds with radial lamellae and intercalation structure for bone regeneration. J Mater Chem B. 2017;5:3640–3650. doi:10.1039/C7TB00485K
Wang K, Ma Q, Zhang YM, Han GT, Qu CX, Wang SD. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose. 2020;27:18745–1852. doi:10.1007/s10570-019-02869-0
Yuan T, Li Z, Zhang Y, Shen K, Zhang X, Xie R, Liu F, Fan W. Injectable ultrasonication-induced silk fibroin hydrogel for cartilage repair and regeneration. Tissue Eng Part A. 2021;27(17–18):1213–1224. doi:10.1089/ten.TEA.2020.0323
Qi Y, Wang H, Wei K, Yang Y, Zheng RY, Kim IS, Zhang KQ. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int J Mol Sci. 2017;18(3):237. doi:10.3390/ijms18030237
Atykyan NA, Revin V, Shutova VV. Raman and FTIR spectroscopy investigation of cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Expr. 2020;10:84. doi:10.1186/s13568-020-01020-8
Barud HGO, Barud HDS, Cavicchioli M, Amaral TSD, Junior OBDO, Santos DM, Petersen ALDOA, Celes F, Borges VM, Oliveira CIDO, Oliveira PFD, Furtado RA, Tavares DC, Ribeiro SJL. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carb Poly. 2015;128:41–51. doi:10.1016/j.carbpol.2015.04.007
Hu Y, Zhang Q, You R, Wang L, Li M. The relationship between secondary structure and biodegradation behavior of silk fibroin scaffolds. Adv Mat Sci Eng. 2012:185905. doi:10.1155/2012/185905
Carissimi G, Baronio CM, Montalban MG, Villora G, Barth A. On the secondary structure of silk fibroin nanoparticles obtained using ionic liquids: an infrared spectroscopy study. Polymers. 2020;12(6):1294. doi:10.3390/polym12061294
DOI: https://doi.org/10.15826/chimtech.2024.11.1.07
Copyright (c) 2024 Mary Stephanie Carranza, Neil Andrew D. Bascos, Maria Carmen Tan, Francisco Franco Jr.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







