Chemical stability aspects of BaCe0.7–xFexZr0.2Y0.1O3–δ mixed ionic-electronic conductors as promising electrodes for protonic ceramic fuel cells
Abstract
Keywords
References
Zainon AN, Somalu MR, Kamarul Bahrain AM, Muchtar A, Baharuddin NA, et al. Challenges in using perovskite-based anode materials for solid oxide fuel cells with various fuels: a review. Int J Hydrogen Energy. 2023;48:20441–20464. doi:10.1016/j.ijhydene.2022.12.192
Su H, Hu YH. Progress in low-temperature solid oxide fuel cells with hydrocarbon fuels. Chem Eng J. 2020;402:126235. doi:10.1016/j.cej.2020.126235
Liu Y, Shao Z, Mori T, Jiang SP. Development of nickel-based cermet anode materials in solid oxide fuel cells – Now and future. Mater Rep Energy. 2021;1:100003. doi:10.1016/j.matre.2020.11.002
Singh M, Zappa D, Comini E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int J Hydrogen Energy. 2021;46:27643–74. doi:10.1016/j.ijhydene.2021.06.020
Hossain MK, Chanda R, El-Denglawey A, Emrose T, Rahman MT, Biswas MC, et al. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram Int. 2021;47:23725–23748. doi:10.1016/j.ceramint.2021.05.167
Nur Syafkeena MA, Zainor ML, Hassan OH, Baharuddin NA, Othman MHD, Tseng C-J, et al. Review on the preparation of electrolyte thin films based on cerate-zirconate oxides for electrochemical analysis of anode-supported proton ceramic fuel cells. J Alloys Compd. 2022;918:165434. doi:10.1016/j.jallcom.2022.165434
Kasyanova AV, Zvonareva IA, Tarasova NA, Bi L, Medvedev DA, Shao Z. Electrolyte materials for protonic ceramic electrochemical cells: Main limitations and potential solutions. Mater Rep Energy. 2022:100158. doi:10.1016/j.matre.2022.100158
Zvonareva IA, Medvedev DA. Proton-conducting barium stannate for high-temperature purposes: A brief review. J Eur Ceram Soc. 2023;43:198–207. doi:10.1016/j.jeurceramsoc.2022.10.049
Wei Z, Wang J, Yu X, Li Z, Zhao Y, Chai J. Study on Ce and Y co-doped BaFeO3–δ cubic perovskite as free-cobalt cathode for proton-conducting solid oxide fuel cells. Int J Hydrogen Energy. 2021;46:23868–23878. doi:10.1016/j.ijhydene.2021.04.188
Tarutin AP, Filonova EA, Ricote S, Medvedev DA, Shao Z. Chemical design of oxygen electrodes for solid oxide electrochemical cells: A guide. Sustain Energy Technol Ass. 2023;57:103185. doi:10.1016/j.seta.2023.103185
Yang L, Ren X, Peng W, Wang A, Yan D, Li J, et al. Triple-conducting Zn-doped Pr1.8Ba0.2NiO4+δ air electrodes for proton ceramic electrolysis cells. J Power Sources. 2023;586:233652. doi:10.1016/j.jpowsour.2023.233652
Chen L, Jing J, Lun P, Zhang P, Zheng Z, Wang H, et al. Ba0.9Co0.7Fe0.2Nb0.1O3–δ perovskite as promising cathode material for proton ceramic fuel cell. Int J Hydrogen Energy. 2023. doi:10.1016/j.ijhydene.2023.07.041
Pei Y, Wang H, Gong J, Yan Z, Xu L, Liu X, et al. Co and Hf co-doped BaFeO3 cathode with obviously enhanced catalytic activity and CO2 tolerance for solid oxide fuel cell. Int J Hydrogen Energy. 2022;47:37945–37955. doi:10.1016/j.ijhydene.2022.08.283
Liu H, Zhu K, Liu Y, Li W, Cai L, Zhu X, et al. Structure and electrochemical properties of cobalt-free perovskite cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim Acta. 2018;279:224–230. doi:10.1016/j.electacta.2018.05.086
Wang M, Su C, Zhu Z, Wang H, Ge L. Composite cathodes for protonic ceramic fuel cells: Rationales and materials. Composites Part B Eng. 2022;238:109881. doi:10.1016/j.compositesb.2022.109881
Hanif MB, Rauf S, Abadeen Z ul, Khan K, Tayyab Z, Qayyum S, et al. Proton-conducting solid oxide electrolysis cells: Relationship of composition-structure-property, their challenges, and prospects. Matter. 2023;6:1782–1830. doi:10.1016/j.matt.2023.04.013
Yang Q, Lu J, Li C, Tian D, Ding Y, Lu X, et al. Tailoring the electrochemical reduction kinetics of dual-phase BaCe0.5Fe0.5O cathode via incorporating Mo for IT-SOFCs. J Eur Ceram Soc. 2023;43:6180–6188. doi:10.1016/j.jeurceramsoc.2023.06.006
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A. 1976;32:751–767. doi:10.1107/S0567739476001551
Dong F, Ni M, He W, Chen Y, Yang G, Chen D, et al. An efficient electrocatalyst as cathode material for solid oxide fuel cells: BaFe0.95SnO3−δ. J Power Sources. 2016;326:459–465. doi:10.1016/j.jpowsour.2016.07.023
Wang J, Lam KY, Saccoccio M, Gao Y, Chen D, Ciucci F. Ca and In co-doped BaFeO3–δ as a cobalt-free cathode material for intermediate-temperature solid oxide fuel cells. J Power Sources. 2016;324:224–232. doi:10.1016/j.jpowsour.2016.05.089
Cui J, Wang J, Zhang X, Li G, Wu K, Cheng Y, et al. Low thermal expansion material Bi0.5Ba0,5FeO3–δ δ in application for proton-conducting ceramic fuel cells cathode. Int J Hydrogen Energy. 2019;44:21127–21135. doi:10.1016/j.ijhydene.2019.02.127
Raimondi G, Merkle R, Longo A, Giannici F, Mathon O, Sahle CJ, et al. Interplay of chemical, electronic, and structural effects in the triple-conducting BaFeO3 –Ba(Zr,Y)O3 solid solution. Chem Mater. 2023;35: 8945–8957. doi:10.1021/acs.chemmater.3c01538
Zhu X, Wang H, Yang W. Structural stability and oxygen permeability of cerium lightly doped BaFeO3−δ ceramic membranes. Solid State Ionics. 2006;177:2917–2921. doi:10.1016/j.ssi.2006.08.027
He W, Fan J, Zhang H, Chen M, Sun Z, Ni M. Zr doped BaFeO3–δ as a robust electrode for symmetrical solid oxide fuel cells. Int J Hydrogen Energy. 2019;44:32164–32169. doi:10.1016/j.ijhydene.2019.10.091
Akbari-Fakhrabadi A, Fábrega G, Ochoa P, Meruane V, Valenzuela P, Gacitúa W. Effect of La3+ and Nb5+on structural and mechanical properties of BaFeO33–δ. J Eur Ceram Soc. 2023;43:6162–6169. doi:10.1016/j.jeurceramsoc.2023.05.017
Wang Z, Wang Y, Wang J, Song Y, Robson MJ, Seong A, et al. Rational design of perovskite ferrites as high-performance proton-conducting fuel cell cathodes. Nat Catal. 2022;5:777–787. doi:10.1038/s41929-022-00829-9
Hu H, Lu Y, Zhou X, Li J, Wang X, Ding X. A/B-site co-doping enabled fast oxygen reduction reaction and promoted CO2 tolerance of perovskite cathode for solid oxide fuel cells. J Power Sources. 2022;548:232049. doi:10.1016/j.jpowsour.2022.232049
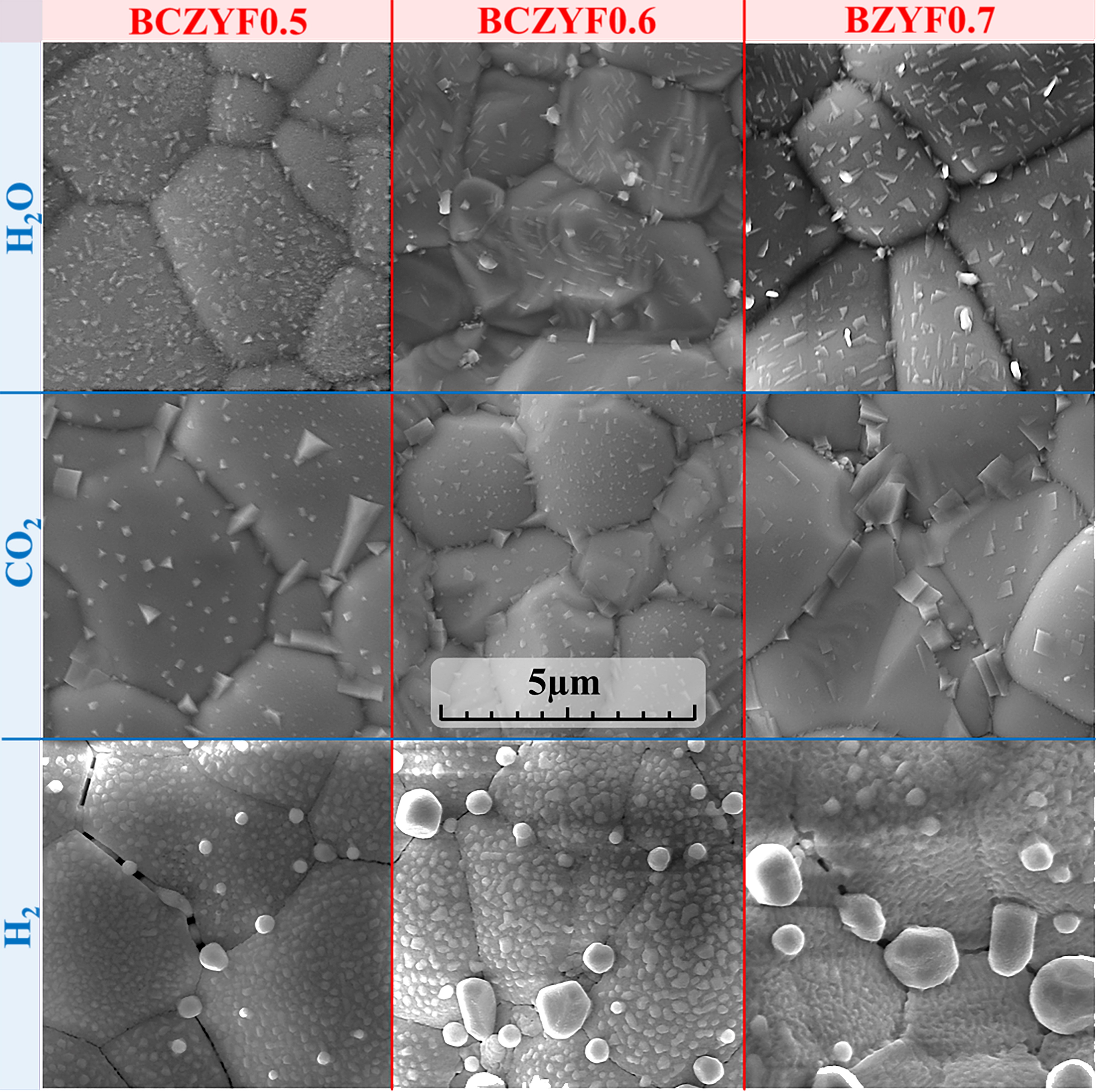
Tarutina LR, Vdovin GK, Lyagaeva JG, Medvedev DA. BaCe0.7–xZr0.2Y0.1FexO3–δ derived from proton-conducting electrolytes: A way of designing chemically compatible cathodes for solid oxide fuel cells. J Alloys Compd. 2020;831:154895. doi:10.1016/j.jallcom.2020.154895
Tarutina LR, Kasyanova AV, Starostin GN, Vdovin GK, Medvedev DA. Electrochemical activity of original and infiltrated Fe-doped Ba(Ce,Zr,Y)O3-based electrodes to be used for protonic ceramic fuel cells. Catalysts. 2022;12:1421. doi:10.3390/catal12111421
Tarutina LR, Vdovin GK, Lyagaeva JG, Medvedev DA. Comprehensive analysis of oxygen transport properties of a BaFe0.7Zr0.2Y0.1O3–δ-based mixed ionic-electronic conductor. J Membr Sci. 2021;624:119125. doi:10.1016/j.memsci.2021.119125
FullProf Suite. Crystallographic tools for Rietveld, profile matching & integrated intensity refinements of X-Ray and/or neutron data [Internet]. https://www.ill.eu/sites/fullprof/
Shen P, Luo J, Zuo Y, Yan Z, Zhang K. Effect of La-Ni substitution on structural, magnetic and microwave absorption properties of barium ferrite. Ceram Int. 2017;43:4846–4851. doi:10.1016/j.ceramint.2016.12.107
Patel CD, Dhruv PN, Meena SS, Singh C, Kavita S, Ellouze M, et al. Influence of Co4+–Ca2+ substitution on structural, microstructure, magnetic, electrical and impedance characteristics of M-type barium–strontium hexagonal ferrites. Ceram Int. 2020;46:24816–24830. doi:10.1016/j.ceramint.2020.05.326
Xian H, Zhang X, Li X, Zou H, Meng M, Zou Z, et al. Effect of the calcination conditions on the NOx storage behavior of the perovskite BaFeO3−x catalysts. Catal Today. 2010;158:215–219. doi:10.1016/j.cattod.2010.03.026
Zhao WY, Wei P, Wu XY, Wang W, Zhang QJ. Lattice vibration characterization and magnetic properties of M-type barium hexaferrite with excessive iron. J Appl Phys. 2008;103. doi:10.1063/1.2884533
Shen P, Luo J, Zuo Y, Yan Z, Zhang K. Effect of La-Ni substitution on structural, magnetic and microwave absorption properties of barium ferrite. Ceram Int. 2017;43:4846–4851. doi:10.1016/j.ceramint.2016.12.107
Ahmad N, Alam M, Adil SF, Ansari AA, Assal ME, Ramay SM, et al. Synthesis, characterization, and selective benzyl alcohol aerobic oxidation over Ni-loaded BaFeO3 mesoporous catalyst. J King Saud Univ Sci. 2020;32:2059–2068. doi:10.1016/j.jksus.2020.02.015
Thomas J, Anitha PK, Thomas T, Thomas N. BaZrO3 based non enzymatic single component single step ceramic electrochemical sensor for the picomolar detection of dopamine. Ceram Int. 2022;48:7168–7182. doi:10.1016/j.ceramint.2021.11.278
Aarthi U, Babu KS. Grain boundary space charge modulation in BaZr0.8Y0.2–xMxO3−δ with transition metal (M= Ni, Co, Fe, and Zn) co-doping. Int J Hydrogen Energy. 2020;45:29356–29366. doi:10.1016/j.ijhydene.2020.07.207
Charoonsuk T, Vittayakorn N. Soft-mechanochemical synthesis of monodispersed BaZrO3 sub-microspheres: Phase formation and growth mechanism. Mater Design. 2017;118:44–52. doi:10.1016/j.matdes.2017.01.029
Fassbender RU, de Carvalho Teixeira V, Galante D, Ferrer M, Jardim PLG, Ratmann CR, et al. Correlation between local structure and electronic properties of BaZrO3:TbYb Optical Ceramics. J Electron Spectrosc Relat Phenomena. 2021;251:147106. doi:10.1016/j.elspec.2021.147106
Triviño-Peláez Á, Mosa J, Pérez-Coll D, Aparicio M, Mather GC. Low-temperature sintering and enhanced stability of fluorine-modified BaZr0.8Y0.2O3–δ synthesised by a sol-gel alkoxide route. J Eur Ceram Soc. 2023;43:99–108. doi:10.1016/j.jeurceramsoc.2022.09.042
DOI: https://doi.org/10.15826/chimtech.2023.10.4.14
Copyright (c) 2023 Liana Tarutina, Inna Starostina, Gennady Vdovin, Svetlana Pershina, Emma Vovkotrub, Anna Murashkina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







