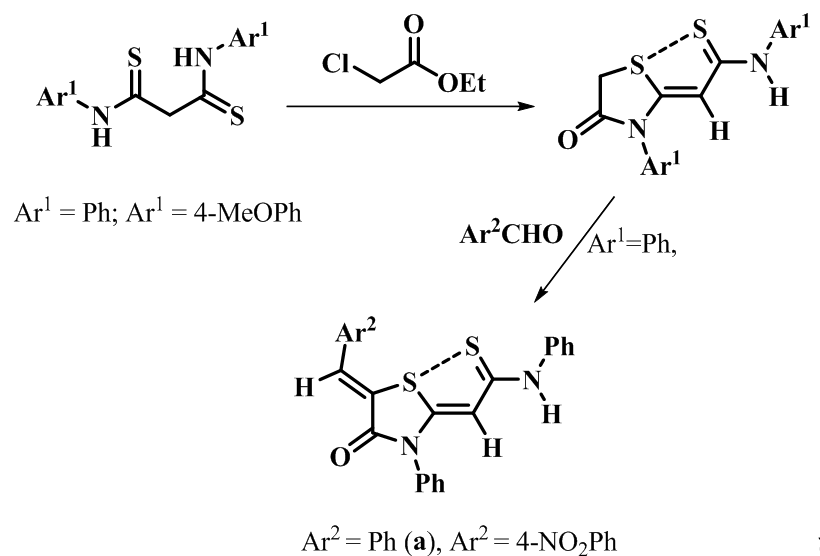
Synthesis of 2-(5-arylidene-4-oxo-3-arylthiazolydine-2)-N-phenylethanthiamides derivatives from malondithioamides
Abstract
Keywords
Full Text:
PDF (RUSSIAN) (Русский)References
Varanasi PR, Jen AK-Y, Chandrasekhar J, Namboothiri INN, Rathna A. The important role of heteroaromatics in the design of efficient second-order nonlinear optical molecules: Theoretical investigation on push-pull heteroaromatic stilbenes. J Am Chem Soc. 1996;118(49):12443-8. doi:10.1021/ja960136q
Ellinger S, Graham KR, Shi P, Farley RT, Steckler TT, Brookins RN,Taranekar P, Mei, J, Padilha LA, Ensley TR, Hu H, Webster S, Hagan DJ, Van Stryland EW, Schanze KS, Reynolds JR. Donor-acceptor-donor-based π-conjugated oligomers for nonlinear optics and near-IR emission. Chem Mater. 2011;23(17):3805-17. doi:10.1021/cm201424a
Andersson AS, Diederich F, Nielsen MB. Acetylenic Tetrathiafulvalene-dicyanovinyl donor-acceptor chromophores. Org Biomol Chem. 2009;7(17):3474-80. doi:10.1039/b909886k
Kivala M, Diederich F. Acetylene-derived strong organic acceptors for planar and nonplanar push-pull chromophores. Acc Chem Res. 2009;42(2):235-48. doi:10.1021/ar8001238
Mahuteau-Betzer F, Piguel S. Synthesis and evaluation of photophysical properties of Series of π-conjugated oxazole dyes. Tetrahedron Lett. 2013;54(24):3188-93. doi:10.1016/j.tetlet.2013.04.037
Facchetti A, Abbotto A, Beverina L, van der Boom ME, Dutta P, Evmenenko G, Marks TJ, Pagani GA. Azinium-(π-bridge)-pyrrole NLO-phores: Influence of heterocycle acceptors on chromophoric and self-assembled thin-film properties. Chem Mater. 2002;14(12):4996-5005. doi:10.1021/cm0205635
Insuasty A, Ortiz A, Tigreros A, Solarte E, Insuasty B, Martín N. 2-(1,1-dicyanomethylene)rhodanine: A novel, efficient electron acceptor. Dyes Pigm. 2011;88(3):385-90. doi:10.1016/j.dyepig.2010.08.11
Baranac-Stojanović M, Kleinpeter E. Quantification of the aromaticity of 2-alkylidenethiazolines subjected to push-pull activity. J Org Chem. 2011;76(10):3861-71. doi:10.1021/jo200294f
Baranac-Stojanović M, Klaumünzer U, Marković R, Kleinpeter E. Structure, configuration, conformation and quantification of the push-pull effect of 2-alkylidene-4-thiazolidinones and 2-alkylidene-4,5-fused bicyclic thiazolidine derivatives. Tetrahedron. 2010;66(46):8958-67. doi:10.1016/j.tet.2010.09.040
Insuasty B, Insuasty A, Tigreros A, Quiroga J, Abonia R, Nogueras M, Cobo J, Derita M, Zacchino S. Synthesis and antifungal evaluation of novel dicyanoderivatives of rhodanine. J Heterocycl Chem. 2011;48(2):347-50. doi:10.1002/jhet.565
Galán E, Andreu R, Garín J, Mosteo L, Orduna J, Villacampa B, Diosdado BE. Influence of thiazole regioisomerism on second-order nonlinear optical chromophores. Tetrahedron. 2012;68(32):6427-34. doi:10.1016/j.tet.2012.05.123
Danilkina NA, Mikhailov LE, Ivin BA. Condensations of thioamides with acetylenecarboxylic acid derivatives. Russ J Org Chem. 2006;42(6):783-811. doi:10.1034/S1070428006060017
Britsun VN, Esipenko AN, Lozinskii MO. Heterocyclization of thioamides containing an active methylene group. Chem Heterocycl Compd. 2008;44(12):1429-59. doi:10.1007/s10593-009-0214-x
Dyachenko VD, Chernega AN, Dyachenko SV. Transamination of cyanothioacetamide with morpholine. molecular and crystal structure of 3-(morpholin-1-yl)-3-thioxopropanenitrile and 3-(morpholin-1-yl)- 3-thioxopropanethioamide. Russ J Gen Chem. 2012;82(4):720-4. doi:10.1134/s1070363212040184
DOI: https://doi.org/10.15826/chimtech.2014.1.2.715
Copyright (c) 2014 K. L. Obydennov, N. A. Golovko, Yu. Yu. Morzherin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






