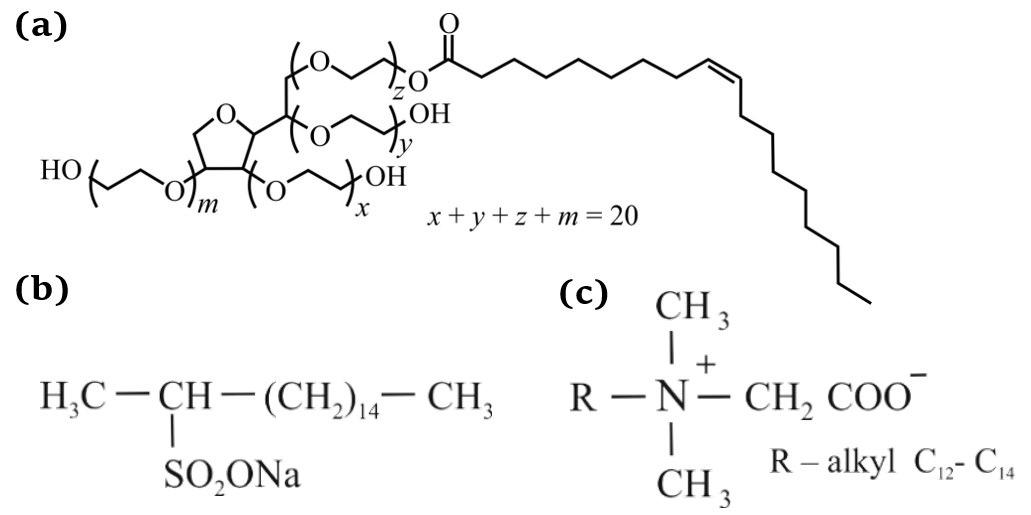
Colloid-chemical properties of surfactant–nitric acid–water systems
Abstract
Keywords
Full Text:
PDFReferences
Agrawal A, Kumar V, Pandey BD, Ahu KK. A comprehensive review on the hydro metallurgical process for the production of nickel and copper powders by hydrogen reduction. Mater Res Bull. 2006;41(4):879–892. doi:10.1016/j.materresbull.2005.09.028
Rogozhnikov DA, Shoppert AA, Dizer OA, Karimov KA, Rusalev RE. Leaching kinetics of sulfides from refractory gold concentrates by nitric acid. Metals. 2019;9(4):465. doi:10.3390/met9040465
Xi J, Ji G, Liao Y, Wu Y, Liu Q, Li M. Research on separation and extraction of valuable metals from complex non-ferrous metals resources by high pressure oxygen leaching methodology: a review. J Sustain Metall. 2022;8(1):51–63. doi:10.1007/s40831-022-00502-2
Larrabure G, Rodriguez-Reyes, Juan F. A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process. Miner Eng. 2021;173. doi:10.1016/j.mineng.2021.107194
Santiago RCC, Ladeira ACQ. Reduction of preg-robbing activity of carbonaceous gold ores with the utilization of surface blinding additives. Miner Eng. doi:10.1016/j.mineng.2018.11.029
Yang HY, Qian LU., Song, XL, Dong JK. Research status of carbonaceous matter in carbonaceous gold ores and bio-oxidation pretreatment. T Nonferr Metal Soc. 2013;23(11):3405–3411. doi:10.1016/S1003-6326(13)62881-2
Habashi F. Nitric acid in the hydrometallurgy of sulfides. Proceedings of the EPD Congress. 1999;357–364.
Rogozhnikov D, Karimov K, Shoppert A, Dizer O, Naboichenko S. Kinetics and mechanism of arsenopyrite leaching in nitric acid solutions in the presence of pyrite and Fe(III) ions. Hydrometallurgy. 2021;199:105525. doi:10.1016/j.hydromet.2020.105525
Rusanov, AI, Shchekin, AK. Micelle formation in solutions of surfactants. Lan Publishing House: St. Petersburg; 2022. 612 р. (in Russian).
Mittel KL, Mukherjee P, Prince LM. Micelle formation, solubilization and microemulsions. Mir: Moscow; 1980. 598 p. (in Russian).
Lugovitskaya TN, Danilin LM, Rogozhnikov DA, Mamyachenkov SV. Behavior of some surfactants in a nitric acid environment and prospects for their use in hydrometallurgy. Rus. J Phys Chem. 2023;97(12):1–7. doi:10.31857/S004445372312021X
Poteshnova MV, Zadymova NM. Aqueous solutions of hydroxypropyl cellulose, Tween 80 and their binary mixtures: Colloid-chemical aspects. Colloid J. 2017;79:797–808. doi:10.1134/S1061933X17060126
Kushnazarova RA, Mirgorodskaya AB, Voloshina AD, Lyubina AP, Kuznetsov DM, Lenina OA, Zakharova LY. Binary systems dicarbamate surfactant – Tween 80: aggregation behavior, antimicrobial activity and membranotropic properties. Liq Cryst Their Appl. 2022;22(2):6–18. doi:10.18083/LCAppl.2022.2.6
Knoch H, Ulbrich MH, Mittag JJ, Buske J, Garidel P, Heerklotz H. Complex micellization behavior of the polysorbates Tween 20 and Tween 80. Mol Pharm. 2021;18(8):3147–3157. doi:10.1021/acs.molpharmaceut.1c00406
Baena-Nogueras RM, Rojas-Ojeda P, Sanz JL, Gonzalez-Mazo E, Lara-Martin PA. Reactivity and fate of secondary alkane sulfonates (SAS) in marine sediments. Environ Pollut. 2014;189:35–42. doi:10.1016/j.envpol.2014.02.019
Garcia MT, Campos E, Marsal A, Ribosa I. Biodegradability and toxicity of sulphonate-based surfactants in aerobic and anaerobic aquatic environments. Water research. 2009;43(2):295–302. doi:10.1016/j.watres.2008.10.016
Wołowicz A, Staszak K. Study of surface properties of aqueous solutions of sodium dodecyl sulfate in the presence of hydrochloric acid and heavy metal ions. J Mol Liq. 2020;299:112170. doi:10.1016/j.molliq.2019.112170
Dey J, Ismail K. Aggregation of sodium dodecylsulfate in aqueous nitric acid medium. J Colloid Interf Sci. 2012;378(1):144–151. doi:10.1016/j.jcis.2012.04.015
Weers JG, Rathman JF, Axe FU, Crichlow CA, Foland LD, Scheuing DR, Wiersema RJ, Zielske AG. Effect of the intramolecular charge separation distance on the solution properties of betaines and sulfobetaines. Langmuir. 1991;7(5):854–867. doi:10.1021/la00053a008
Dukhin A, Parlia S. Ions, ion pairs and inverse micelles in non-polar media. Curr Opin Сolloid Interface Sci. 2013;18(2):93–115. doi:10.1016/j.cocis.2013.02.004
DOI: https://doi.org/10.15826/chimtech.2023.10.4.08
Copyright (c) 2023 Lev Danilin, Tatyana Lugovitskaya, Elvira Kolmachikhina, Denis Rogozhnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







