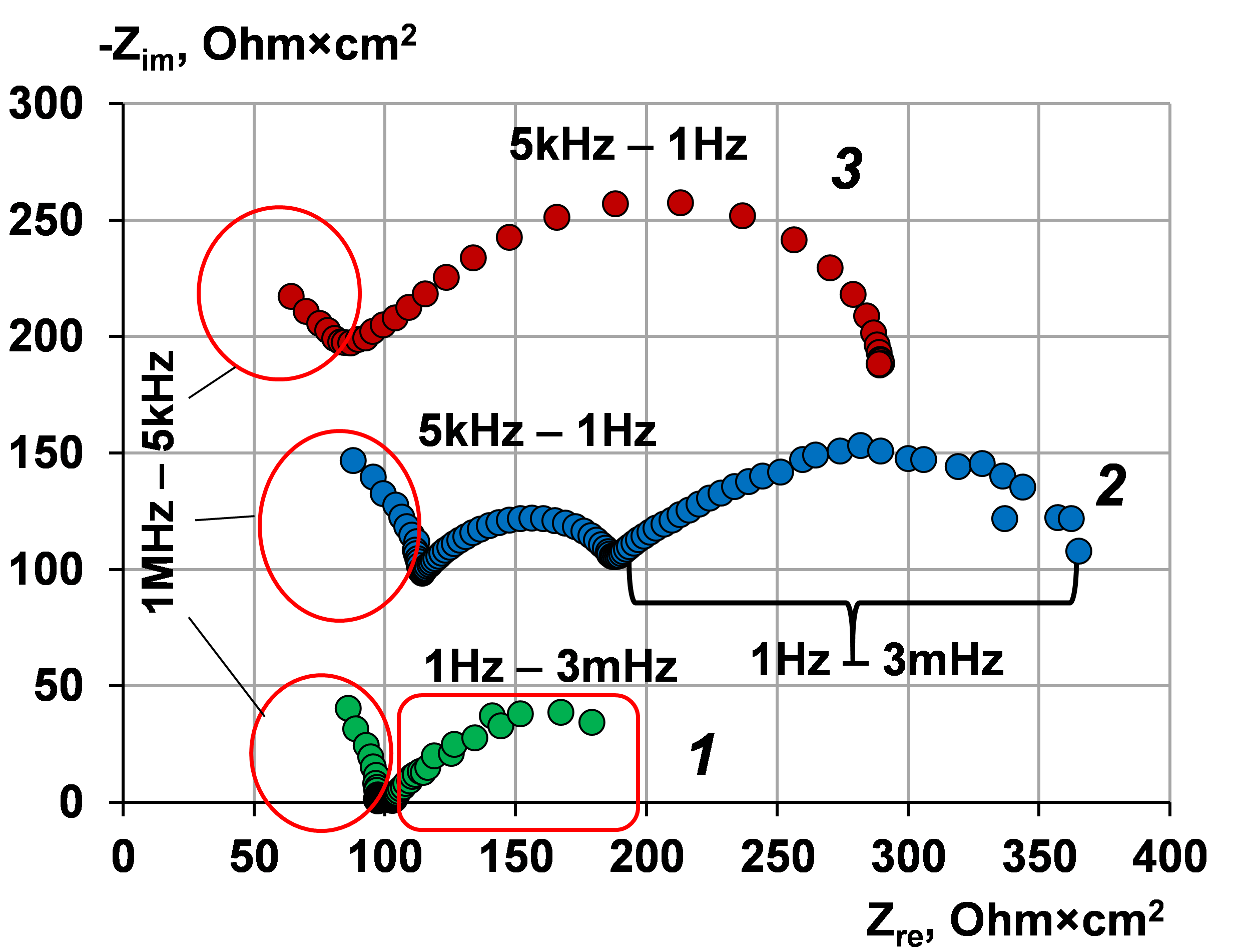
Use of electrochemical impedance spectroscopy to assess the stability of the anion exchange membrane MA-41, modified by poly-N,N-diallylmorpholine bromide in overlimiting current modes
Abstract
Keywords
Full Text:
PDFReferences
Strathmann H. Electrodialysis, a mature technology with a multitude of new applications. Desalination. 2010;264:268–288. doi:10.1016/j.desal.2010.04.069
Urtenov MK, Uzdenova AM, Kovalenko AV, Nikonenko VV, Pismenskaya ND, Vasil’eva VI, Sistat P, Pourcelly G. Basic mathematical model of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells. J Membr Sci. 2013;447:190–202. doi:10.1016/j.memsci.2013.07.033
Nikonenko VV, Kovalenko AV, Urtenov MK, Pismenskaya ND, Han J, Sistat P, Pourcelly G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination. 2014;342:85–106. doi:10.1016/j.desal.2014.01.008
Zabolotskiy VI, But AY, Vasil’eva VI, Akberova EM, Melnikov SS. Ion transport and electrochemical stability of strongly basic anion-exchange membranes under high current electrodialysis conditions. J Membr Sci. 2017;526:60–72. doi:10.1016/j.memsci.2016.12.028
Nikonenko VV, Mareev SA, Pis’menskaya ND, Uzdenova AM, Kovalenko AV, Urtenov MK, Pourcelly G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis. Russ J Electrochem. 2017;53:1122–1144. doi:10.1134/S1023193517090099
Bauer B, Strathmann H, Effenberger F. Anion-exchange membranes with improved alkaline stability. Desalination. 1990;79:125–144. doi:10.1016/0011-9164(90)85002-R
Akberova EM, Malyhin MD. Strukturnye i fiziko-himicheskie harakteristiki anionoobmennyh membran MA-40 i MA-41 posle termohimicheskogo vozdejstviya [Structural and physico-chemical characteristics of anion-exchange membranes MA-40 and MA-41 after thermochemical exposure. Sorption and chromatographic processes]. Sorbcionnye i hromatograficheskie processy. 2014;14(2):232–239. Russian.
Shaposhnik VA, Kastyuchik AS, Kozaderova OA. Irreversible dissociation of water molecules on the ion-exchange membrane-electrolyte solution interface in electrodialysis. Russ J Electrochem. 2008;44:1073–1077. doi:10.1134/S1023193508090139
Loza SA, Smyshlyaev NA, Korzhov AN, Romanyuk NA. Electrodialysis concentration of sulfuric acid. Chim Techno Acta. 2021;8(1):20218106. doi:10.15826/chimtech.2021.8.1.06
Andreeva MA, Gil VV, Pismenskaya ND, Nikonenko VV, Dammak L, Larchet C, Grande D, Kononenko NA. Effect of homogenization and hydrophobization of a cation-exchange membrane surface on its scaling in the presence of calcium and magnesium chlorides during electrodialysis. J Membr Sci. 2017;540:183–191. doi:10.1016/j.memsci.2017.06.030
Pismenskaya ND, Belova EI, Nikonenko VV, Zabolotsky VI, Lopatkova GY, Karzhavin Y N, Larchet C. Lower rate of H+(OH–) ions generation at an anion-exchange membrane in electrodialysis. Desalination Water Treat. 2010;21:109–114. doi:10.5004/dwt.2010.1268
Slouka Z, Senapati S, Yan Y, Chang HC. Charge inversion, water splitting, and vortex suppression due to DNA sorption on ion-selective membranes and their ion-current signatures. Langmuir. 2013;29:8275–8283. doi:10.1021/la4007179
Bondarev D, Melnikov S, Zabolotskiy V. New homogeneous and bilayer anion-exchange membranes based on N, N-diallyl-N, N-dimethylammonium chloride and ethyl methacrylate copolymer. J Membr Sci. 2023;675:121510. doi:10.1016/j.memsci.2023.121510
Knyaginicheva EV, Belashova ED, Pis'menskaya ND. Elektrohimicheskie harakteristiki membrany AMH, modificirovannoj sil'nymi bifunkcional'nymi polielektrolitami. [Electrochemical characteristics of the AMX membrane modified with strong bifunctional polyelectrolytes.] Sorbcionnye i hromatograficheskie processy. 2014;14(5):864–868. Russian.
Butylskii DY, Troitskiy VA, Sharafan MV, Pismenskaya ND, Nikonenko VV. Scaling-resistant anion-exchange membrane prepared by in situ modification with a bifunctional polymer containing quaternary amino groups. Desalination. 2022;537:115821. doi:10.1016/j.desal.2022.115821
Marino MG, Kreuer KD. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem. 2015;8:513–523. doi:10.1002/cssc.201403022
Cotanda P, Petzetakis N, Jiang X, Stone G, Balsara NP. Hydroxide-ion transport and stability of diblock copolymers with a polydiallyldimethyl ammonium hydroxide block. J Polym Sci Part A Polym Chem. 2017;55(13):2243–2248. doi:10.1002/pola.28611
Olsson JS, Pham TH, Jannasch P. Poly (N,N-diallylazacycloalkane) s for anion-exchange membranes functionalized with N-spirocyclic quaternary ammoniumcation. Macromolec. 2017;50:2784–2793. doi:10.1021/acs.macromol.7b00168
Pham TH, Olsson JS, Jannasch P. Poly (arylene alkylene) s with pendant N-spirocyclic quaternary ammonium cations for anion exchange membranes. J Mater Chem A. 2018;6:16537–16547. doi:10.1039/C8TA04699A
Zabolotskii VI, Bondarev DA, Bespalov AV. Electrochemical and mass transport characteristics of the strongly basic MA-41 membrane modified by poly-N,N-diallylmorpholinium. Russ J Electrochem. 2018;54:963–969. doi:10.1134/S1023193518130529
Krol JJ, Wessling M, Strathmann H. Concentration polarization with monopolar ion exchange membranes: current-voltage curves and water dissociation. J Membr Sci. 1999;162:145–154. doi:10.1016/S0376-7388(99)00133-7
Nikonenko SV, Urtenov MK, Kovalenko AV, Semenchin EA, Nikonenko VV. Meaning of the diffusion coefficient in Peers equation for calculating limiting current density. Numerical analysis results. Condensed Matter and Interphases. 2011;13(3):320–326. https://journals.vsu.ru/kcmf/article/view/1059
Sistat P, Kozmai A, Pismenskaya N, Larchet C, Pourcelly G, Nikonenko V. Low-frequency impedance of an ion-exchange membrane system. Electrochim Acta. 2008;53(22):6380–6390. doi:10.1016/j.electacta.2008.04.041
Zabolotsky VI, Novak L, Kovalenko AV, Nikonenko VV, Urtenov MH, Lebedev KA, But AY. Electroconvection in systems with heterogeneous ion-exchange membranes. Petrol Chem. 2017;57:779–789. doi:10.1134/S0965544117090109
Pismenskaya ND, Pokhidnia EV, Pourcelly G, Nikonenko VV. Can the electrochemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes? J Membr Sci. 2018;566.54–68. doi:10.1016/j.memsci.2018.08.055
Gelferix F. Ionity Osnovy ionnogo obmena [Ionites. Basics of ion exchange]. Moscow: Izdat. Inostran. Lit; 1962. 491 p. Russian.
Demina OA, Demin AV, Gnusin NP, Zabolotskii VI. Effect of an aprotic solvent on the properties and structure of ion-exchange membranes. Polymer Sci Ser A. 2010;52(12):1270–1282. doi:10.1134/S0965545X10120059
Kniaginicheva E, Pismenskaya N, Melnikov S, Belashova E, Sistat P, Cretin M, Nikonenko V. Water splitting at an anion-exchange membrane as studied by impedance spectroscopy. J Membr Sci. 2015;496:78–83. doi:10.1016/j.memsci.2015.07.050
Pismenskaya ND, Rybalkina OA, Kozmai AE, Tsygurina KA, Melnikova ED, Nikonenko VV. Generation of H+ and OH− ions in anion-exchange membrane/ampholyte-containing solution systems: A study using electrochemical impedance spectroscopy. J Membr Sci. 2020;601:117920. doi:10.1016/j.memsci.2020.117920
Barsoukov E, Macdonald JR. Impedance spectroscopy: theory, experiment, and applications, second ed., New Jersey: John Wiley & Sons; 2005. 560 p. doi:10.1002/0471716243
Barbero G. Warburg’s impedance revisited. Phys. Chem. Chem. Phys. 2016;18:29537–29542. doi:10.1039/C6CP05049B
Zabolockij VI, Shel'deshov NV, Gnusin NP. Dissociaciya molekul vody v sistemah s ionoobmennymi membranami [Dissociation of water molecules in systems with ion-exchange membranes.]. Uspekhi himii. 1988;57(8):1403–1414. Russian. doi:10.1070/RC1988v057n08ABEH003389
Hurwitz HD, Dibiani R. Experimental and theoretical investigations of steady and transient states in systems of ion exchange bipolar membranes. J Membr Sci. 2004;228:17–43. doi:10.1016/j.memsci.2003.09.009
Umnov VV, Shel’deshov NV, Zabolotskii VI. Current-voltage curve for the space charge region of a bipolar membrane. Russ J Electrochem. 1999;35:871–878.
DOI: https://doi.org/10.15826/chimtech.2023.10.4.04
Copyright (c) 2023 Denis A. Bondarev, Aslan R. Achoh, Alexander V. Bespalov, Stanislav S. Melnikov, Mikhail V. Sharafan, Viktor I. Zabolotskiy

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







