The most effective techniques of industrial purification processes: a technical review
Abstract
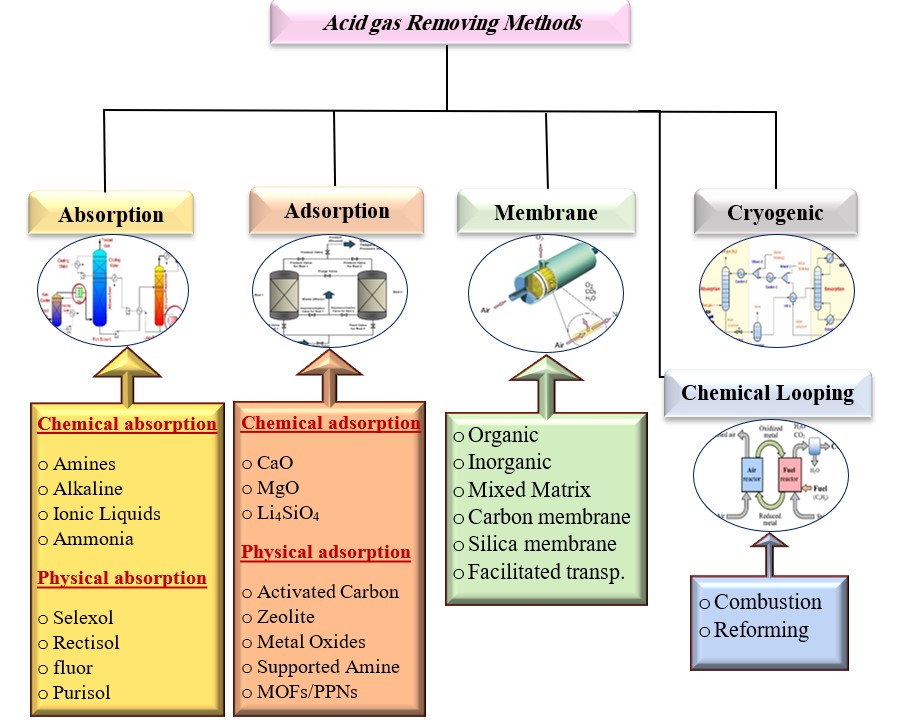
This paper reviews various separation techniques used in purification processes to remove pollutants like carbon dioxide and hydrogen sulfide from petroleum products. The most effective techniques include absorption, adsorption, cryogenic distillation, chemical looping combustion, and membrane separation. The study reviews over 100 published studies to assess their characteristics, benefits, and drawbacks. The choice of separation technology depends on ideal conditions, cost, efficiency, and energy required in the regeneration phase.
Keywords
Full Text:
PDFReferences
Suleman H, Fosbøl PL, Nasir R, Ameen M. Sustainable carbon capture: technologies and applications. Boca Ra-ton CRC Press. 2022. doi:10.1201/9781003162780
Araújo OQF, Reis AC, Medeiros JL, do Nascimento JF, Grava WM, Musse APS. Comparative analysis of separa-tion technologies for processing carbon dioxide rich natural gas in ultra-deepwater oil fields. J Clean Prod. 2017; 155:12–22. doi:10.1016/j.jclepro.2016.06.073
Egging-Bratseth R. A techno-economic perspective on natural gas and its value chain. Gases. 2020;1(1):1–18. doi:10.3390/gases1010001
Hafeez S, et al. CO2 capture using membrane contactors: a systematic literature review. Front Chem Sci Eng. 2021;15(4):720–754. doi:10.1007/s11705-020-1992-z
Kazmi B, Raza F, Taqvi SAA, Awan ZH, Ali SI, Suleman H. Energy, exergy and economic (3E) evaluation of CO2 capture from natural gas using pyridinium functional-ized ionic liquids: A simulation study. J Nat Gas Sci Eng. 2021;90:103951. doi:10.1016/j.jngse.2021.103951
Yamada H. Amine-based capture of CO2 for utilization and storage. Polym J. 2021;53(1):93–102. doi:10.1038/s41428-020-00400-y
Shafiq U, Shariff AM, Babar M, Azeem B, Ali A, Bustam A. Study of dry ice formation during blowdown of CO₂-CH₄ from cryogenic distillation column. J Loss Prev Pro-cess Ind. 2020;64:104073. doi:10.1016/j.jlp.2020.104073
Podder J, Patra BR, Pattnaik F, Nanda S, Dalai AK. A re-view of carbon capture and valorization technologies. Energies. 2023;16(6):1–29. doi:10.3390/en16062589
Peres CB, Resende PMR, Nunes LJR, Morais LCD. Ad-vances in carbon capture and use (CCU) technologies: a comprehensive review and CO2 Mitigation potential analysis. Clean Technol. 2022;4(4):1193–1207. doi:10.3390/cleantechnol4040073
Liu F, Rochelle GT, Fang M, Wang T. Volatility of 2-(diethylamino)-ethanol and 2-((2-aminoethyl) amino) ethanol, a biphasic solvent for CO2 capture. Int J Greenh Gas Control. 2021; 106:103257. doi:10.1016/j.ijggc.2021.103257
Lin Q, Zhang X, Wang T, Zheng C, Gao X. Technical per-spective of carbon capture, utilization, and storage. Engin. 2022; 14:27–32. doi:10.1016/j.eng.2021.12.013
Madejski P, Chmiel K, Subramanian N, Kus T. Methods and techniques for CO2 capture: review of potential. En-ergies. 2022; 15:887. doi:10.3390/en15030887
Zhang Y, et al. Functional biochar synergistic sol-id/liquid-phase CO2 capture: a review. Energy Fuels. 2022;36(6):2945–2970. doi:10.1021/acs.energyfuels.1c04372
Dashti H, Zhehao Yew L, Lou X. Recent advances in gas hydrate-based CO2 capture. J Nat Gas Sci Eng. 2015;23:195–207. doi:10.1016/j.jngse.2015.01.033
Wang M, Lawal A, Stephenson P, Sidders J, Ramshaw C. Post-combustion CO2 capture with chemical absorption: a state-of-the-art review. Chem Eng Res Des. 2011;89(9):1609–1624. doi:10.1016/j.cherd.2010.11.005
Osman AI, Hefny M, Abdel Maksoud MIA, Elgarahy AM, Rooney DW. Recent advances in carbon capture storage and utilisation technologies: a review. Springer Int Publ. 2021;19(2). doi:10.1007/s10311-020-01133-3
Georgiadis AG, Charisiou ND, Goula MA. Removal of hydrogen sulfide from various industrial gases: A re-view of the most promising adsorbing materials. Cata-lysts. 2020;10(5). doi:10.3390/catal10050521
Artanto Y, et al. Pilot-scale evaluation of AMP/PZ to cap-ture CO2 from flue gas of an Australian brown coal-fired power station. Int J Greenh Gas Control. 2014; 20:189–195. doi:10.1016/j.ijggc.2013.11.002
Shunji K, Xizhou S, Wenze Y. Investigation of CO2 de-sorption kinetics in MDEA and MDEA+DEA rich amine solutions with thermo-gravimetric analysis method. Int J Greenh Gas Control. 2020; 95:102947. doi:10.1016/j.ijggc.2019.102947
Idem R, et al. Pilot plant studies of the CO2 capture per-formance of aqueous MEA and mixed MEA/MDEA sol-vents at the University of Regina CO2 capture technolo-gy development plant and the boundary dam CO2 cap-ture demonstration plant. Ind Eng Chem Res. 2006;45(8):2414–2420. doi:10.1021/ie050569e
Abdulrahman RK, Sebastine IM. Natural gas sweetening process simulation and optimization: A case study of Khurmala field in Iraqi Kurdistan region. J Nat Gas Sci Eng. 2013; 14:116–120. doi:10.1016/j.jngse.2013.06.005
Liu J, Wang S, Zhao B, Tong H, Chen C. Absorption of carbon dioxide in aqueous ammonia. Energy Proc. 2009;1(1):933–940. doi:10.1016/j.egypro.2009.01.124
Zhu D, Fang M, Zhong L, Zhang C, Luo Z. Semi-batch experimental study on CO2 absorption characteristic of aqueous ammonia. Energy Proc. 2011;4:156–163. doi:10.1016/j.egypro.2011.01.036
iang, K., Yu, H., Chen, L., Fang, M., Azzi, M., Cottrell, A., & Li, K. “An advanced, ammonia-based combined NOx/SOx/CO2 emission control process towards a low-cost, clean coal technology,” Appl. Energy, vol. 260, p. 114316, 2020, doi:10.1016/j.apenergy.2019.114316
Pahija E, Golshan S, Blais B, Boffito DC. Perspectives on the process intensification of CO2 capture and utiliza-tion. Chem Eng Process Intensif. 2022; 176:108958. doi:10.1016/j.cep.2022.108958
Mejía I, Stanley K, Canales R, Brennecke JF. On the high-pressure solubilities of carbon dioxide in several ionic liquids. J Chem Eng Data. 2013;58(9):2642–2653. doi:10.1021/je400542b
Dang LX, Wick CD. Anion effects on interfacial absorp-tion of gases in ionic liquids. Molecular dynamics study. J Phys Chem B. 2011;115(21):6964–6970. doi:10.1021/jp201113c
Bates ED, Mayton RD, Ntai I, Davis JH. CO2 capture by a task-specific ionic liquid. J Am Chem Soc. 2002;124(6):926–927. doi:10.1021/ja017593d
Zaman M, Lee JH. Carbon capture from stationary power generation sources: A review of the current status of the technologies. Korean J Chem Eng. 2013;30(8):1497–1526. doi:10.1007/s11814-013-0127-3
Gunawardene OHP, Gunathilake CA, Vikrant K, Ama-raweera SM. Carbon dioxide capture through physical and chemical adsorption using porous carbon materials: A review. Atmosphere (Basel). 2022;13(3):397. doi:10.3390/atmos13030397
Aquatar MO, Bhatia U, Rayalu SS, Krupadam RJ. Reduced graphene oxide MnO2 nanocomposite for CO2 capture from flue gases at elevated temperatures. Sci Total En-viron. 2022;816:151522. doi:10.1016/j.scitotenv.2021.151522
Maniarasu R, Rathore SK, Murugan S. A review on ma-terials and processes for carbon dioxide separation and capture. Energy Environ. 2023;34(1):3–57. doi:10.1177/0958305X211050984
Cavenati S, Grande CA, Rodrigues AE. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorp-tion for upgrade of natural gas. Chem Eng Sci. 2006;61(12):3893–3906. doi:10.1016/j.ces.2006.01.023
Al-Mamoori A, Krishnamurthy A, Rownaghi AA, Rezaei F. Carbon capture and utilization update. Energy Tech-nol. 2017;5(6):834–849. doi:10.1002/ente.201600747
Jiang, L., Liu, W., Wang, R. Q., Gonzalez-Diaz, A., Rojas-Michaga, M. F., Michailos, S., Pourkashanian, M., Zhang, X. J., & Font-Palma, C .“Sorption direct air capture with CO2 utilization,” Prog. Energy Combust. Sci., vol. 95, p. 101069, 2023, doi:10.1016/j.pecs.2022.101069
Agency IE. Energy technology perspectives 2020-special report on carbon capture utilisation and storage: CCUS in Clean Energy Transitions. OECD Publishing, 2020. doi:10.1787/208b66f4-en
Boot-Handford ME, et al. Carbon capture and storage update. Energy Environ Sci. 2014;7(1):130–189. doi: 10.1201/9781315369853-23
Kuppler RJ, et al. Potential applications of metal-organic frameworks. Coord Chem Rev. 2009;253(23–24):3042–3066. doi:10.1016/j.ccr.2009.05.019
Li J-R, Kuppler RJ, Zhou H-C. Selective gas adsorption and separation in metal–organic frameworks. Chem Soc Rev. 2009;38(5):1477–1504. doi:10.1039/B802426J
Song C, Liu Q, Deng S, Li H, Kitamura Y. Cryogenic-based CO2 capture technologies: State-of-the-art devel-opments and current challenges. Renew Sustain Energy Rev. 2019; 101:265–278. doi:10.1016/j.rser.2018.11.018
Brunetti A, Scura F, Barbieri G, Drioli E. Membrane technologies for CO2 separation. J Memb Sci. 2010;359(1–2):115–125. doi:10.1016/j.memsci.2009.11.040
Shen M, et al. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep Purif Technol. 2022; 299:121734. doi:10.1016/j.seppur.2022.121734
Bi Y, Ju Y. Review on cryogenic technologies for CO2 removal from natural gas. Front Energy. 2022:1–19. doi:10.1007/s11708-022-0821-0
Knapik E, Kosowski P, Stopa J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem Eng Res Des. 2018;131:66–79. doi:10.1016/j.cherd.2017.12.027
Abuelgasim S, Wang W, Abdalazeez A. A brief review for chemical looping combustion as a promising CO2 cap-ture technology: Fundamentals and progress. Sci Total Environ. 2021;764:142892. doi:10.1016/j.scitotenv.2020.142892
Liu G, Lisak G. Cu-based oxygen carriers for chemical looping processes: Opportunities and challenges. Fuel. 2023;342:127828. doi:10.1016/j.fuel.2023.127828
Daneshmand-Jahromi S, Sedghkerdar MH, Mahinpey N. A review of chemical looping combustion technology: Fundamentals, and development of natural, industrial waste, and synthetic oxygen carriers. Fuel. 2023;341:127626. doi:10.1016/j.fuel.2023.127626
Liu F, Liu J, Yang Y. Review on the theoretical under-standing of oxygen carrier development for chemical-looping technologies. Energy Fuels. 2022;36(17):9373–9384. doi:10.1021/acs.energyfuels.2c00961
Ozcan DC, et al. Ca-Cu looping process for CO2 capture from a power plant and its comparison with Ca-looping, oxy-combustion and amine-based CO2 capture processes. Int J Greenh Gas Control. 2015;43:198–212. doi:10.1016/j.ijggc.2015.10.021
Abuelgasim S, Wang W, Abdalazeez A. A brief review for chemical looping combustion as a promising CO2 cap-ture technology: Fundamentals and progress. Sci Total Environ. 2020:142892. doi:10.1016/j.scitotenv.2020.142892
Yaqub ZT, Oboirien BO, Leion H. Chemical looping com-bustion (CLC) of municipal solid waste (MSW). J Mater Cycles Waste Manag. 2023;25(4):1900–1920. doi:10.1007/s10163-023-01674-z
Goel A, Moghaddam EM, Liu W, He C, Konttinen J. Bio-mass chemical looping gasification for high-quality syngas: A critical review and technological outlooks. Energy Convers Manag. 2022; 268:116020. doi:10.1016/j.enconman.2022.116020
Norahim N, Yaisanga P, Faungnawakij K, Charinpa-nitkul T, Klaysom C. Recent membrane developments for CO2 separation and capture. Chem Eng Technol. 2018;41(2):211–223. doi:10.1002/ceat.201700406
Zach B, Pluskal J, Šomplák R, Jadrný J, Šyc M. Tool for optimization of energy consumption of membrane-based carbon capture. J Environ Manage. 2022; 320:115913. doi:10.1016/j.jenvman.2022.115913
Chen T-Y, Deng X, Lin L-C, Ho WSW. New sterically hin-dered polyvinylamine-containing membranes for CO2 capture from flue gas. J Memb Sci. 2022; 645:120195. doi:10.1016/j.memsci.2021.120195
Ahmad NNR, Ang WL, Leo CP, Mohammad AW, Hilal N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desal-ination. 2021; 517:115170. doi:10.1016/j.desal.2021.115170
Schlumberger transition technologies case study: natu-ral gas processing Petronas gas plant reduces costs with dual-core acid gas removal membranes. 2022. https://www.slb.com/resource-library/case-study/osf/cynara-pn-1-petronas-malaysia-cs
Nogalska A, Trojanowska A, Garcia-Valls R. Membrane contactors for CO2 capture processes – Critical review. Phys Sci Rev. 2019;2(7). doi:10.1515/psr-2017-0059
Cui Y, Zhao Y, Wang T, Han B. Benzimidazole-linked porous polymers: Synthesis and gas sorption properties. Chinese J Chem. 2015;33(1):131–136. doi:10.1002/cjoc.201400494
Genduso G, Ghanem BS, Pinnau I. Experimental mixed-gas permeability, sorption and diffusion of CO2-CH4 mix-tures in 6FDA-mPDA polyimide membrane: Unveiling the effect of competitive sorption on permeability selec-tivity. Membranes (Basel). 2019;9(1):10. doi:10.3390/membranes9010010
Zhang Y, Sunarso J, Liu S, Wang R. Current status and development of membranes for CO2/CH4 separation: A review. Int J Greenh Gas Control. 2013; 12:84–107. doi:10.1016/j.ijggc.2012.10.009
Yerzhankyzy A, Wang Y, Ghanem BS, Puspasari T, Pin-nau I. Gas separation performance of solid-state in-situ thermally crosslinked 6FDA-based polyimides. J Memb Sci. 2022;641:119885. doi:10.1016/j.memsci.2021.119885
Liu Z, Liu Y, Qiu W, Koros WJ. Molecularly engineered 6FDA-based polyimide membranes for sour natural gas separation. Angew Chemie Int Ed. 2020;59(35):14877–14883. doi:10.1002/anie.202003910
Farrokhara M, Dorosti F. New high permeable polysul-fone/ionic liquid membrane for gas separation. Chinese J Chem Eng. 2020;28(9):2301–2311. doi:10.1016/j.cjche.2020.04.002
Porcheron F, et al. Hollow fiber membrane contactors for CO2 capture: From lab-scale screening to pilot-plant module conception. Energy Procedia. 2011;4:763–770. doi:10.1016/j.egypro.2011.01.117
Fashandi H, Ghodsi A, Saghafi R, Zarrebini M. CO2 ab-sorption using gas-liquid membrane contactors made of highly porous poly(vinyl chloride) hollow fiber mem-branes. Int J Greenh Gas Control. 2016;52:13–23. doi:10.1016/j.ijggc.2016.06.010
Fu H, Xue K, Li Z, Zhang H, Gao D, Chen H. Study on the performance of CO2 capture from flue gas with ceramic and PTFE membrane contactors. Energy. 2023;263. doi:10.1016/j.energy.2022.125677
Lawal SO, Yu L, Nagasawa H, Tsuru T, Kanezashi M. A carbon-silica-zirconia ceramic membrane with CO2 flow-switching behaviour promising versatile high-temperature H2/CO2 separation. J Mater Chem A. 2020;8(44):23563–23573. doi:10.1039/d0ta07065c
Zhu M, et al. Influences of acid post-treatment on high silica SSZ-13 zeolite membrane. Ind Eng Chem Res. 2019;58(31):14037–14043. doi:10.1021/acs.iecr.9b01250
Li G, et al. Permeation characteristics of a T-type zeolite membrane for bio-oil pervaporation dehydration. Mi-croporous Mesoporous Mater. 2021;315:110884. doi:10.1016/j.micromeso.2021.110884
Pagliero M, Bottino A, Comite A, Costa C. Novel hydro-phobic PVDF membranes prepared by nonsolvent in-duced phase separation for membrane distillation. J Memb Sci. 2020;596:117575. doi:10.1016/j.memsci.2019.117575
Lee HJ, Kim MK, Park JH, Magnone E. Temperature and pressure dependence of the CO2 absorption through a ceramic hollow fiber membrane contactor module. Chem Eng Process Process Intensif. 2020;150:107871. doi:10.1016/j.cep.2020.107871
Kim S, Scholes CA, Heath DE, Kentish SE. Gas-liquid membrane contactors for carbon dioxide separation: A review. Chem Eng J. 2021;411:128468. doi:10.1016/j.cej.2021.128468
Lee Y, Park Y-J, Lee J, Bae T-H. Recent advances and emerging applications of membrane contactors. Chem Eng J. 2023;461:141948. doi:10.1016/j.cej.2023.141948
Zainuddin MIF, Ahmad AL. Mixed matrix membrane development progress and prospect of using 2D nanosheet filler for CO2 separation and capture. J CO2 Util. 2022;62:102094. doi:10.1016/j.jcou.2022.102094
Nord LO, Bolland O. Carbon dioxide emission manage-ment in power generation. John Wiley & Sons, 2020. doi:10.1002/9783527826667
Goh SH, Lau HS, Yong WF. Metal–organic frameworks (MOFs)‐based mixed matrix membranes (MMMs) for gas separation: a review on advanced materials in harsh environmental applications. Small. 2022;18(20):2107536. doi:10.1002/smll.202107536
Chao C, Deng Y, Dewil R, Baeyens J, Fan X. Post-combustion carbon capture. Renew Sustain Energy Rev. 2021;138:110490. doi:10.1016/j.rser.2020.110490
Tai ZS, et al. Development of hydrophobic polymethylhydrosiloxane/tetraethylorthosilicate (PMHS/TEOS) hybrid coating on ceramic membrane for desalination via membrane distillation. J Memb Sci. 2021;637:119609. doi:10.1016/j.memsci.2021.119609
Alftessi SA, et al. Omniphobic surface modification of silica sand ceramic hollow fiber membrane for desali-nation via direct contact membrane distillation. Desali-nation. 2022;532:115705. doi:10.1016/j.desal.2022.115705
Gong H, Pang H, Du M, Chen Z. Fabrication of a super-hydrophobic mixed matrix PVDF-SiO2-HDTMS hollow fi-ber membrane for membrane contact carbon dioxide ab-sorption. Clean Eng Technol. 2021;5:100278. doi:10.1016/j.clet.2021.100278
Lin YF, Kuo JW. Mesoporous bis(trimethoxysilyl)hexane (BTMSH)/tetraethyl orthosilicate (TEOS)-based hybrid silica aerogel membranes for CO2 capture. Chem Eng J. 2016;300:29–35. doi:10.1016/j.cej.2016.04.119
Abdulhameed MA, et al. Carbon dioxide capture using a superhydrophobic ceramic hollow fibre membrane for gas-liquid contacting process. J Clean Prod. 2017;140:1731–1738. doi:10.1016/j.jclepro.2016.07.015
Zhang X, Song Z, Gani R, Zhou T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind Eng Chem Res. 2020;59(5):2005–2012. doi:10.1021/acs.iecr.9b05510
Fang M, Yi N, Di W, Wang T, Wang Q. Emission and control of flue gas pollutants in CO2 chemical absorp-tion system – A review. Int J Greenh Gas Control. 2020;93:102904. doi:10.1016/j.ijggc.2019.102904
Madejski P, Chmiel K, Subramanian N, Kuś T. Methods and techniques for CO2 capture: review of potential so-lutions and applications in modern energy technologies. Energies. 2022;15(3):887. doi:10.3390/en15030887
Elhambakhsh A, Keshavarz P. Effects of different amin-based core-shell magnetic NPs on CO2 capture using NMP solution at high pressures. J Nat Gas Sci Eng. 2020;84:103645. doi:10.1016/j.jngse.2020.103645
Ochedi FO, Yu J, Yu H, Liu Y, Hussain A. Carbon dioxide capture using liquid absorption methods: a review. En-viron Chem Lett. 2021;19(1):77–109. doi:10.1007/s10311-020-01093-8
Khalifa O, Alkhatib III, Bahamon D, Alhajaj A, Abu-Zahra MRM, Vega LF. Modifying absorption process con-figurations to improve their performance for Post-Combustion CO2 capture – What have we learned and what is still Missing? Chem Eng J. 2022;430:133096. doi:10.1016/j.cej.2021.133096
Patel HA, Byun J, Yavuz CT. Carbon dioxide capture ad-sorbents: chemistry and methods. ChemSusChem. 2017;10(7):1303–1317. doi:10.1002/cssc.201601545
Lam MK, Lee KT, Mohamed AR. Current status and chal-lenges on microalgae-based carbon capture. Int J Greenh Gas Control. 2012;10:456–469. doi:10.1016/j.ijggc.2012.07.010
Carchini G, Hussein I, Al-Marri M. J, Shawabkeh R, Apa-ricio S. A theoretical study of gas adsorption on calcite for CO2 enhanced natural gas recovery. 3rd EAGE WIPIC Work. Reserv Manag Carbonates. 2019;504:144575. doi:10.3997/2214-4609.201903135
Raganati F, Chirone R, Ammendola P. CO2 Capture by temperature swing adsorption: working capacity as af-fected by temperature and CO2 partial pressure. Ind Eng Chem Res. 2020;59(8):3593–3605. doi:10.1021/acs.iecr.9b04901
Hussin F, Aroua MK. Recent trends in the development of adsorption technologies for carbon dioxide capture: A brief literature and patent reviews (2014–2018). J Clean Prod. 2020;253:119707. doi:10.1016/j.jclepro.2019.119707
Babar M, Bustam MA, Maulud AS, Ali A, Mukhtar A, Ul-lah S. Enhanced cryogenic packed bed with optimal CO2 removal from natural gas; a joint computational and experimental approach. Cryogenics (Guildf). 2020;105:103010. doi:10.1016/j.cryogenics.2019.103010
Pellegrini LA, De Guido G, Ingrosso S. Thermodynamic framework for cryogenic carbon capture. in computer aided chemical engineering. Elsevier. 2020:475–480. doi:10.1016/B978-0-12-823377-1.50080-X
Sukor NR, Shamsuddin AH, Mahlia TMI, Isa MFM. Tech-no-economic analysis of CO2 capture technologies in offshore natural gas field: Implications to carbon cap-ture and storage in Malaysia. Processes. 2020;8(3):350. doi:10.3390/pr8030350
Yan Y, Wang K, Clough PT, Anthony EJ. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: A review. Fuel Process Technol. 2020;199:106280. doi:10.1016/j.fuproc.2019.106280
Osman M, Khan MN, Zaabout A, Cloete S, Amini S. Re-view of pressurized chemical looping processes for pow-er generation and chemical production with integrated CO2 capture. Fuel Process Technol. 2021;214:106684. doi:10.1016/j.fuproc.2020.106684
Lyngfelt A. Chemical looping combustion: status and development challenges. Energy Fuels. 2020;34(8):9077–9093. doi:10.1021/acs.energyfuels.0c01454
Khan MN, Chiesa P, Cloete S, Amini S. Integration of chemical looping combustion for cost-effective CO2 cap-ture from state-of-the-art natural gas combined cycles. Energy Convers Manag X. 2020;7:100044. doi:10.1016/j.ecmx.2020.100044
Shah C, Raut S, Kacha H, Patel H, Shah M. Carbon cap-ture using membrane-based materials and its utiliza-tion pathways. Chem Pap. 2021;75(9):4413–4429. doi:10.1007/s11696-021-01674-z
Sanni ES, Sadiku ER, Okoro EE. Novel systems and membrane technologies for carbon capture. Int J Chem Eng. 2021. doi:10.1155/2021/6642906
Patil T, Dharaskar S, Sinha M, Jampa SS. Effectiveness of ionic liquid-supported membranes for carbon dioxide capture: a review. Environ Sci Pollut Res. 2022;29(24):35723–35745. doi:10.1007/s11356-022-19586-0
Kammerer S, Borho I, Jung J, Schmidt MS. Review: CO2 capturing methods of the last two decades. Int J Environ Sci Technol. 2023;20(7):8087–8104. doi:10.1007/s13762-022-04680-0
DOI: https://doi.org/10.15826/chimtech.2023.10.4.03
Copyright (c) 2023 Iltifat Hameed Saud, Forat Yasir AlJaberi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







