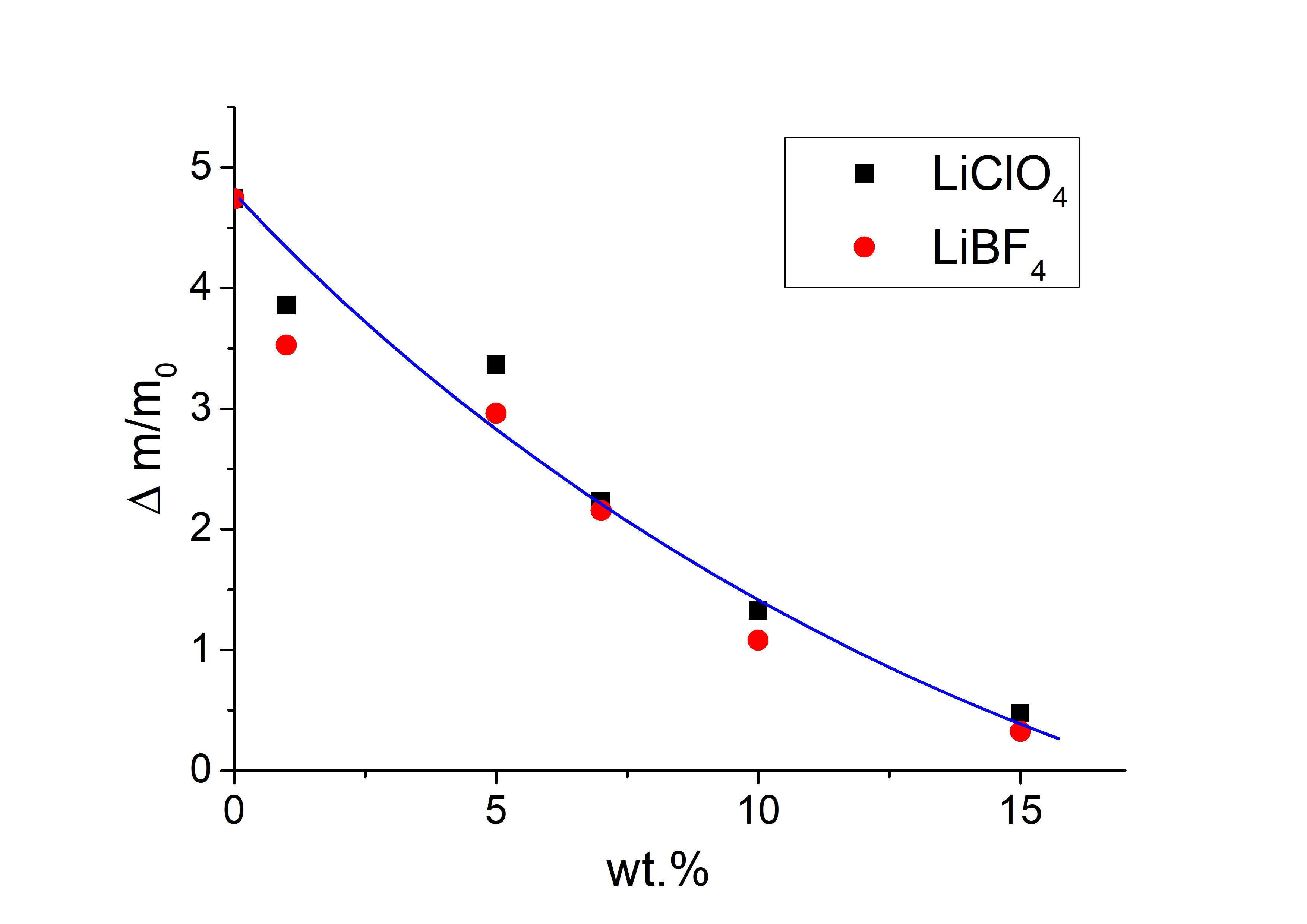
Synthesis and properties of polymer electrolytes based on polyurethane elastomer and lithium salts
Abstract
Keywords
Full Text:
PDFReferences
Armand M, Tarascon JM. Building better batteries. Nat. 2008;451(7179):652–657. doi:10.1038/451652a
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid a battery of choices. Sci. 2011;334(6058):928–935. doi:10.1126/science1212741
Zhou Q, Ma J, Dong S, Li X, Cui G. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv Mater. 2019;31(50):1902029. doi:10.1002/adma.201902029
Zhang D, Meng X, Hou W, Hu W, Mo J, Yang T, Zhang W, Fan Q, Liu L, Jiang B, Chu L, Li M. Solid polymer electrolytes Ion conduction mechanisms and enhancement strategies. Nano Res Energy. 2023;2:e9120050. doi:10.26599/NRE.2023.9120050
Agrawal RC, Pandey GP. Solid polymer electrolytes: materials designing and all-solid-state battery applications an overview. Phys. 2008;41(22):223001. doi:10.1088/0022-3727/41/22/223001
Quartarone E, Mustarelli P. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev. 2011;40:2525–2540. doi:10.1039/C0CS00081G
Rosero D, Meneses J, Uribe-Kaffure R. Composite polymer electrolytes based on (PEO)4CF3COOLi and multi-walled carbon nanotube (MWCNT). Polym. 2023;15(1):49. doi:10.3390/polym15010049
Sun C, Liu J, Gong Y, Wilkinson D, Zhang J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy. 2017;33:363–386. doi:10.1016/j.nanoen.2017.01.028
Francielli S, Barna J, Wang J, Biria S, Ian D. Solid polymer electrolyte from photo-crosslinked polytetrahydrofuran and a cycloaliphatic epoxide for lithium-ion conduction. MRS Advances. 2020;5:2467–2476. doi:10.1557/adv.2020.274
Zhao H, Zhang W, Yin X, Wu Y, Du C, Zhao W, Zhaoab L, Liu C. Conductive polyurethane elastomer electrolyte (PUEE) materials for anodic bonding. RSC Advances. 2020;10:13267–13276. doi:10.1039/c9ra10944g
Gupta NV, Shivakumar HG. Investigation of swelling behavior and mechanical properties of a pH-sensitive superporous hydrogel composite. Iran J Pharmac Res. 2012;11(2):481–493 doi:10.22037/IJPR.2012.1097
Victorov A, Radke C, Prausnitz J. Molecular thermodynamics for swelling of a mesoscopic ionomer gel in 1:1 salt solutions. Phys Chem Chem Phys. 2006;8:264–278. doi:10.1039/b512748c
Miguel I, Rosero D, Nori M, Meneses J, Uribe Kaffure R. Thermal properties of composite polyme relectrolytes poly(ethylene oxide) sodium trifluoroacetate aluminum oxide (PEO)10CF3COONa + x wt.% Al2O3. Mater. 2019;12(9):1464. doi:10.3390/ma12091464
Dimitriyev MS, Chang YW, Goldbart PM, Nieves AF. Swelling thermodynamics and phase transitions of polymer gels. Nano Futures. 2019;3(4):042001. doi:10.1088/2399-1984/ab45d5
Wilcox KG, Kozawa SK, Morozova S. Fundamentals and mechanics of polyelectrolyte gels: Thermodynamics, swelling, scattering, and elasticity. Chem Phys Rev. 2021;2(4):041309 doi:10.1063/5.0048152
Okumura D, Chester SA. Ultimate swelling described by limiting chain extensibility of swollen elastomers. Int J Mechan Sci. 2018;144:531–539. doi:10.1016/j.ijmecsci.2018.06.011
Yu Y, Landis C.M, Huang R. Salt-Induced Swelling and Volume Phase Transition of Polyelectrolyte Gels. J Appl Mechanics. 2017;84(5):051005. doi:10.1115/1.4036113
Kou R, Zhang J, Wang T, Liu G. Interactions between polyelectrolyte brushes and hofmeister ions chaotropes versus kosmotropes. Langmuir. 2015;31(38):10461−10469. doi:10.1021/acs.langmuir.5b02698
Son H, Woo HS, Park VS, Min JY, Kim DW. Polyurethane-based elastomeric polymer electrolyte for lithium metal polymer cells with enhanced thermal safety. Electrochem Soc. 2020;167(8):080525. doi:10.1149/1945-7111/ab8ed2
Zhang Q, Wena Y, Liu K, Liu N, Du Y, Mac C, Zhou L, Liang Y, Jin Y. Study of solid polyurethane electrolytes synthesized from HDI and PEO of different molecular weight. Electroanal Chem.2021;893:115305. doi:10.1016/j.jelechem.2021.115305
DOI: https://doi.org/10.15826/chimtech.2023.10.3.11
Copyright (c) 2023 Nikita Fedorov, Artem Ulihin, Nikolai Uvarov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







