Influence of anionic surfactant on stability of nanoparticles in aqueous solutions
Abstract
Keywords
Full Text:
PDFReferences
Alsaba MT, Al Dushaishi MF, Abbas AK. A comprehensive review of nanoparticles applications in the oil and gas industry. J Pet Explor Prod Technol. 2020;10(5):1389–1399. doi:10.1007/s13202-019-00825-z
Franco CA, Zabala R, Cortés FB. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J Pet Sci Eng. 2017;157:39–55. doi:10.1016/j.petrol.2017.07.004
Chen L, Zhu X, Wang L, Yang H, Wang D, Fu M. Experimental study of effective amphiphilic graphene oxide flooding for an ultralow-permeability reservoir. Energy Fuels. 2018;32(11):11269–11278. doi:10.1021/acs.energyfuels.8b02576
Goshtasp C. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Petroleum Sci Technol. 2016;34(7):633–641. doi:10.1080/10916466.2016.1156125
Dai C, Li H, Zhao M, Wu Y, You Q, Sun Y, Zhao G, Xu K. Emulsion behavior control and stability study through decorating silica nano-particle with dimethyldodecylamine oxide at n-heptane/water interface. Chem Eng Sci. 2018;179:73–82. doi:10.1016/j.ces.2018.01.005
Eltoum H, Yang YL, Hou JR. The effect of nanoparticles on reservoir wettability alteration: a critical review. Petroleum Sci. 2021;18:136–153. doi:10.1007/s12182-020-00496-0
Karimi A, Fakhroueian Z, Bahramian A, Khiabani NP, Darabad JB, Azin R, Arya S. Wettability alteration in carbonates using zirconium oxide nanofluids: EOR Implications. Energy Fuels. 2012;26(2):1028–1036. doi:10.1021/ef201475u
Fan H, Striolo A. Nanoparticle effects on the water-oil interfacial tension. Phys Rev E. 2012;86(5):051610. doi:10.1103/PhysRevE.86.051610
Almahfood M, Bai B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J Petroleum Sci Eng. 2018;171:196–210. doi:10.1016/j.petrol.2018.07.030
Zhong X, Li C, Li Y, Pu H, Zhou Y, Zhao J. Enhanced oil recovery in high salinity and elevated temperature conditions with a zwitterionic surfactant and silica nanoparticles acting in synergy. Energy Fuels. 2020;34(3):2893–2902. doi:10.1021/acs.energyfuels.9b04067
Arab D, Kantzas A, Bryant SL. Nanoparticle stabilized oil in water emulsions: A critical review. J Pet Sci Eng. 2018;163:217–242. doi:10.1016/j.petrol.2017.12.091
Behzadi A, Mohammadi A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J Nanopart Res. 2016;18:1–19. doi:10.1007/s11051-016-3580-1
Ngouangna EN, Manan MA, Oseh JO, Norddin MNA, Agi A, Gbadamosi AO. Influence of (3–Aminopropyl) triethoxysilane on silica nanoparticle for enhanced oil recovery. J Mol Liquids. 2020;315:113740. doi:10.1016/j.molliq.2020.113740
Zhao T, Chen J, Chen Y, Zhang Y, Peng J. Study on synergistic enhancement of oil recovery by halloysite nanotubes and glucose-based surfactants. J Dispersion Sci Technol. 2021;42:934–946. doi:10.1080/01932691.2020.1721297
Son HA, Lee T. Enhanced oil recovery with size-dependent interactions of nanoparticles surface-modified by zwitterionic surfactants. Appl Sci. 2021;11(16):7184. doi:10.3390/app11167184
Ahmed A, Saaid IM, Ahmed AA, Pilus RM, Baig MK. Evaluating the potential of surface-modifed silica nanoparticles using internal olefn sulfonate for enhanced oil recovery. Pet Sci. 2019;17:722–733. doi:10.1007/s12182-019-00404-1
Pal N, Verma A, Ojha K, Mandal A. Nanoparticle-modified gemini surfactant foams as efficient displacing fluids for enhanced oil recovery. J Mol Liquids. 2020;310:113193. doi:10.1016/j.molliq.2020.113193
Venancio JCC, Nascimento RSV, Perez-Gramatges A. Colloidal stability and dynamic adsorption behavior of nanofluids containing alkyl-modified silica nanoparticles and anionic surfactant. J Mol Liquids. 2020;308:1–7. doi:10.1016/j.molliq.2020.113079
Moghadam TF, Azizian S, Wettig S. Synergistic behaviour of ZnO nanoparticles and gemini surfactants on the dynamic and equilibrium oil/water interfacial tension. Phys Chem Chem Phys. 2015;17(11):1–8. doi:10.1039/C5CP00510H
Pillai P, Sawa RK, Singha R, Padmanabhan E, Mandal A. Effect of synthesized lysine-grafted silica nanoparticle on surfactant stabilized O/W emulsion stability: Application in enhanced oil recovery. J Pet Sci Eng. 2019;177:861–871. doi:10.1016/j.petrol.2019.03.007
Saigal T, Dong H, Matyjaszewski K, Tilton RD. Pickering Emulsions Stabilized by Nanoparticles with Thermally Responsive Grafted Polymer Brushes. Langmuir. 2010;26(19):15200–15209. doi:10.1021/la1027898
Dai C, Li H, Zhao M, Wu Y, You Q, Sun Y, Zhao G, Xu K. Emulsion behavior control and stability study through decorating silica nano-particle with dimethyldodecylamine oxide at n-heptane/water interface. Chem Eng Sci. 2018;179:73–82. doi:10.1016/j.ces.2018.01.005
Omran M, Akarri S, Torsaeter O. The effect of wettability and flow rate on oil displacement using polymer-coated silica nanoparticles: a microfluidic study. Proces. 2020;8(8):991. doi:10.3390/pr8080991
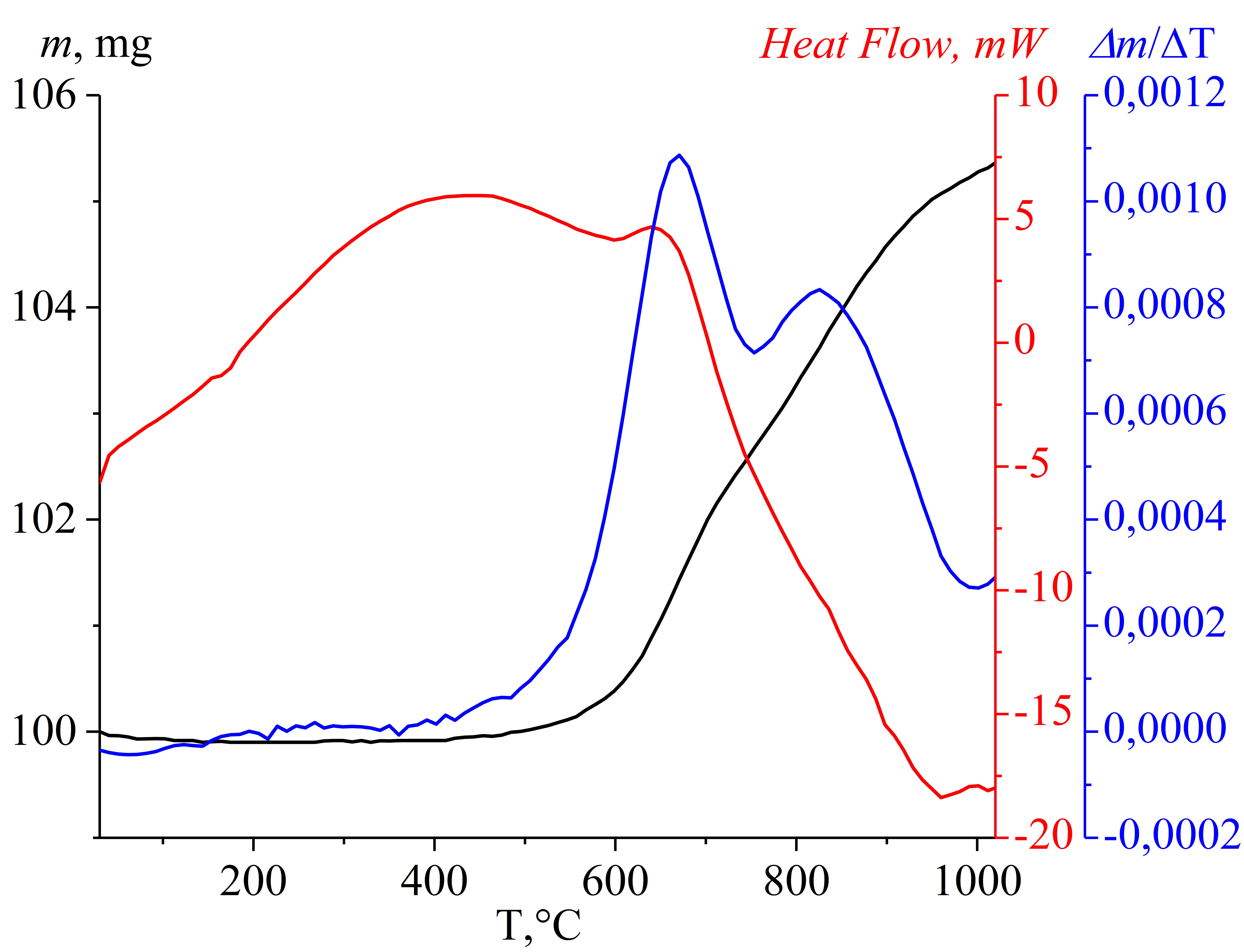
Mishchenko KV, Gerasimov KB, Yukhin YM. Thermal decomposition of some bismuth oxocarboxylates with formation of β-Bi2O3. Mater Today Proc. 2020;25(3):391–394. doi:10.1016/j.matpr.2019.12.102
Pak AY, Povalyaev PV, Frantsina EV, Grinko AA, Petrova YY, Arkachenkova VV. Obtaining carbon graphite-like nanomaterials in asphaltene-based waste recycling. Bulletin of the Tomsk Polytechnic University. Geo Assets Eng. 2022;333(12):25–36. Russian. Available from: https://earchive.tpu.ru/handle/11683/74343
Sivkov A, Vympina Y, Ivashutenko A, Rakhmatullin I, Shanenkova Y, Nikitin D, Shanenkov I. Plasma dynamic synthesis of highly defective fine titanium dioxide with tunable phase composition. Ceram Int. 2022;48(8):10862–10873. doi:10.1016/j.ceramint.2021.12.303
Viades-Trejo J, Gracia-Fadrique J. Spinning drop method: From Young–Laplace to Vonnegut. Colloids Surfaces A Physicochem Eng Aspects. 2007;302(1–3):549–552. doi:10.1016/j.colsurfa.2007.03.033
Cao Y, Zhao RH, Zhang L, Xu ZC, Jin ZQ, Luo L, Zhang L, Zhao S. Effect of electrolyte and temperature on interfacial tensions of alkylbenzene sulfonate solutions. Energy Fuels. 2012;26(4):2175–2181. doi:10.1021/ef201982s
Sharma S, Reddy AVD, Jayarambabu N, Kumar NVM, Saineetha A, Rao KV, Kailasa S. Synthesis and characterization of Titanium dioxide nanopowder for various energy and environmental applications. Mater Today Proc. 2020;26(1):158–161. doi:10.1016/j.matpr.2019.09.203
DOI: https://doi.org/10.15826/chimtech.2023.10.3.02
Copyright (c) 2023 Dmitry O. Zelentsov, Yuliya Yu. Petrova, Alexander V. Korobkin, Anastasia A. Ivanova, Alexey N. Cheremisin, Ivan I. Shanenkov, Alexander Ya. Pak, Yuliya G. Mateyshina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







