The oligosuccinimide and modified polysuccinimide as green corrosion and scale inhibitors
Abstract
Keywords
Full Text:
PDFReferences
Mady MF, Rehman A, Kelland MA. Synthesis and study of modified polyaspartic acid coupled phosphonate and sulfonate moieties as green oilfield scale inhibitors. Ind Eng Chem Res. 2021;60(23):8331–8339. doi:10.1021/acs.iecr.1c01473
Chen Y, Zhou Y, Yao Q, Nan Q, Zhang M, Sun W. Synthesis of modified polyepoxysuccinic acid and evaluation of its scale inhibition on CaCO3, CaSO4, and Ca3(PO4)2 precipitation for industrial recycling water. Desalin Water Treat. 2019;152:16–25. doi:10.5004/dwt.2019.23918
Chai C, Xu Y, Li D, Zhao X, Xu Y, Zhang L. Progress in organic coatings cysteamine modified polyaspartic acid as a new class of green corrosion inhibitor for mild steel in sulfuric acid medium: synthesis, electrochemical, surface study and theoretical calculation. Prog Org Coatings. 2019;129:159–170. doi:10.1016/j.porgcoat.2018.12.028
Wang Q, Liang F, Al-nasser W, Al-dawood F. Laboratory study on efficiency of three calcium carbonate scale inhibitors in the presence of EOR chemicals. Petroleum. 2018;4:375–384. doi:10.1016/j.petlm.2018.03.003
Rahman F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination. 2013;319:79–84. doi:10.1016/j.desal.2013.03.027
Al-Roomi YM, Hussain KF. Potential kinetic model for scaling and scale inhibition mechanism. Desalination. 2016;393:186–195. doi:10.1016/j.desal.2015.07.025
Ali SA, Kazi IW, Rahman F. Synthesis and evaluation of phosphate-free antiscalants to control CaSO4·2H2O scale formation in reverse osmosis desalination plants. Desalination. 2015;357:36–44. doi:10.1016/j.desal.2014.11.006
Qiang AY, Zhang S, Yan S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Eval Program Plann. 2017;126:295–304. doi:10.1016/j.corsci.2017.07.012
Song FM, Kirk DW, Graydon JW, Cormack DE. Predicting carbon dioxide corrosion of bare steel under an aqueous boundary layer. Corrosion. 2004;60(8):736–748. doi:10.5006/1.3287853
Kumar CMP, Chandrashekarappa MPG, Kulkarni RM, Pimenov DY, Giasin K. The effect of Zn and Zn-WO3 composites nano-coatings deposition on hardness and corrosion resistance in steel substrate. Mater (Basel). 2021;14(9):2253. doi:10.3390/ma14092253
Romijarso TB, Rohmah M, Tajalla GUN, Siradj ES, Mabruri E, Synergistic effect of chromium content and intercritical annealing process on corrosion-resistant improvement of Ni-Cr-Mo low alloy. Int J Corros Scale Inhib. 2022;11(4):1557–1568. doi:10.17675/2305-6894-2022-11-4-8
Pfeifer K, Telegdi J. Improved hydrophobicity for better corrosion control by special self-assembled molecular coatings. Int J Corros Scale Inhib. 2022;11(3):1041–1062. doi:10.17675/2305-6894-2022-11-3-9
Makarychev YB, Luchkin AY, Grafov OY, Andreev NN. Vapor-phase deposition of polymer siloxane coatings on the surface of copper and low-carbon steel. Int J Corros Scale Inhib. 2022;11(3):980–1000. doi:10.17675/2305-6894-2022-11-3-6
Arunadevi N, Swathika M, Mehala M, Ranjith Kumar E, Bawazeer TM, Morad M, Alkhamis K, Al-nami SY, El-Metwaly NM. New epoxy-Nano metal oxide-based coatings for enhanced corrosion protection. J Mol Struct. 2022;1250:131790. doi:10.1016/j.molstruc.2021.131790
Bystrov SG, Reshetnikov SM, Borisova, Pisareva, Bayankin VY. Study on the efficiency of benzotriazole and mercaptobenzothiazole as corrosion inhibitors of some high-alloy steels in neutral environment. Int J Corros Scale Inhib. 2022;11(2):647–658. doi:10.17675/2305-6894-2022-11-2-13
Elbadaoui A, Galai M, Ferraa S, Barebita H, Cherkaoui M, Guedira T. A new family of borated glasses as a corrosion inhibitor for steel in 1.0 M hydrochloric acid:synthesis and cauterization studies. Int J Corros Scale Inhib. 2022;11(2):666–685. doi:10.17675/2305-6894-2022-11-2-15
Bedir AG, Abd El-raouf M, Abdel-Mawgoud S, Negm NA, El Basiony NM. Corrosion inhibition of carbon steel in hydrochloric acid solution using ethoxylated nonionic surfactants based on schiff base:electrochemical and computational investigations. ACS Omega. 2021;6(6):4300–4312. doi:10.1021/acsomega.0c05476
Oh D, Zhou L, Chang D, Lee WA. Novel hydrogen peroxide stabilizer in descaling process of metal surface. Chem Eng J. 2018;334:1169–1175. doi:10.1016/j.cej.2017.11.058
Qu Z, Wu L, An Y, Fang R, Jin S, Yang J, Liu Y, Wang L, Yang X, Yan D. A descaling methodology for a water-filled pipe based on leaky guided ultrasonic waves cavitation. Chem Eng Res Des. 2019;146:470–477. doi:10.1016/j.cherd.2019.04.027
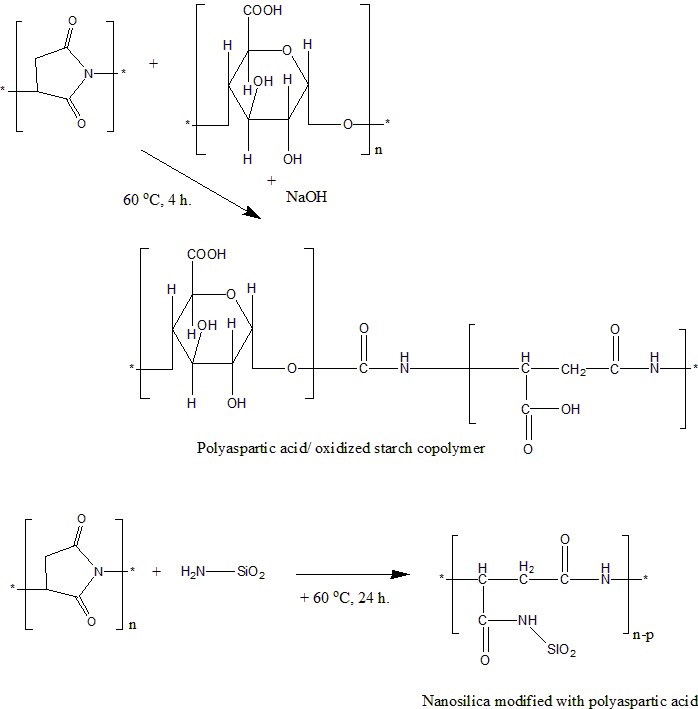
Chen Y, Chen X, Liang Y, Gao Y. Synthesis of polyaspartic acid-oxidized starch copolymer and evaluation of its inhibition performance and dispersion capacity. J Dispers Sci Technol. 2021;42(13):1926–1935. doi:10.1080/01932691.2020.1791172
Baari MJ, Bundjali B, Wahyuningrum D. Performance of N,O-carboxymethyl chitosan as corrosion and scale inhibitors in CO2 saturated brine solution. Indones J Chem. 2021;21(4):954–967. doi:10.22146/ijc.64255
Zheng Y, Gao Y, Li H, Yan M, Zhao J, Liu Z. Chitosan-acrylic acid-polysuccinimide terpolymer as environmentally friendly scale and corrosion inhibitor in artificial seawater. Desalination. 2021;520:115367. doi:10.1016/j.desal.2021.115367
Fu L, Lv J, Zhou L, Li Z, Tang M, Li J. Study on corrosion and scale inhibition mechanism of polyaspartic acid grafted β-cyclodextrin. Mater Lett. 2020;264:127276. doi:10.1016/j.matlet.2019.127276
Zhang Y, Yin H, Zhang Q, Li Y, Yao P, Huo H. A novel polyaspartic acid derivative with multifunctional groups for scale inhibition application. Environ Technol. 2018;39(7):843–850. doi:10.1080/09593330.2017.1312551
Chen Y, Xing W, Wang L, Chen L. Experimental and electrochemical research of an efficient corrosion and scale inhibitor. Mater (Basel). 2019;12(11):1821. doi:10.3390/ma12111821
Li L, Wu J, Zhao M, Wang Y, Zhang H, Zhang X, Gui L, Liu J, Mair N, Peng S. Poly-α,β-DL-aspartyl-L-cysteine:a novel nanomaterial having a porous structure, special complexation capability for Pb(II), and selectivity of removing Pb(II). Chem Res Toxicol. 2012;25(9):1948–1954. doi:10.1021/tx300265c
Shi S, Wu Y, Wang Y, Yu J, Xu Y. Synthesis and characterization of a biodegradable polyaspartic acid/2-amino-2-methyl-1-propanol graft copolymer and evaluation of its scale and corrosion inhibition performance. RSC Adv. 2017;7(58):36714–36721. doi:10.1039/c7ra06848d
Yu W, Wang Y, Li A, Yang H. Evaluation of the structural morphology of starch-graft-poly(acrylic acid) on its scale-inhibition efficiency. Water Res. 2018;141:86–95. doi:10.1016/j.watres.2018.04.021
Baari MJ, Bundjali B, Wahyuningrum D. Synthesis of oligosuccinimide and evaluation of its corrosion inhibition performance on carbon steel in CO2-saturated 1% NaCl solution. J Math Fundam Sci. 2020;52(2):202–221. doi:10.5614/j.math.fund.sci.2020.52.2.5
Liu Z, Sun Y, Zhou X, Wu T, Tian Y, Wang Y. Synthesis and scale inhibitor performance of polyaspartic acid. J Environ Sci. 2011;23:S153–S155. doi:10.1016/S1001-0742(11)61100-5
Sun X, Zhang J, Yin C, Zhang J, Han J. Poly(aspartic acid)-tryptophan grafted copolymer and its scale-inhibition performance. J Appl Polym Sci. 2015;132(45):2–9. doi:10.1002/app.42739
Wang X, Lv X, Zhang B, Xu B, Xu Y. Scale inhibition performance research of polyaspartic acid/diethylenetriamine graft copolymer. J Chem Eng Japan. 2015;48(6):506–510. doi:10.1252/jcej.14we096
Migahed MA, Rashwan SM, Kamel MM, Habib RE. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J Mol Liq. 2016;224:849–858. doi:10.1016/j.molliq.2016.10.091
Zhou Y, Wang J, Fang Y. Green and high effective scale inhibitor based on ring-opening graft modification of polyaspartic acid. Catal. 2021;11(7):802. doi:10.3390/catal11070802
Zhang Y, Yin H, Zhang Q, Li Y, Yao P. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance. Desalination. 2016;395:92–98. doi:10.1016/j.desal.2016.05.020
Zeino A, Abdulazeez I, Khaled M, Jawich MW, Obot IB. Mechanistic study of polyaspartic acid (PASP) as eco-friendly corrosion inhibitor on mild steel in 3% NaCl aerated solution. J Mol Liq. 2018;250:50–62. doi:10.1016/j.molliq.2017.11.160
Piątkowski M, Bogdał D, Raclavský K. 1H and 13C NMR analysis of poly(succinimide) prepared by microwave-enhanced polycondensation of L-aspartic acid. Int J Polym Anal Charact. 2015;20(8):714–723. doi:10.1080/1023666X.2016.1081134
Zhang S, Qu H, Yang Z, Fu CE, Tian Z, Yang W. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination. 2017;419:152–159. doi:10.1016/j.desal.2017.06.016
Zeng D, Chen T, Zhou S. Synthesis of polyaspartic acid/chitosan graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance. Int J Electrochem Sci. 2015;10(11):9513–9527. doi:10.1016/S1452-3981(23)11197-7
Chai C, Xu Y, Xu Y, Liu S, Zhang L. Dopamine-modified polyaspartic acid as a green corrosion inhibitor for mild steel in acid solution. Eur Polym J. 2020;137:109946. doi:10.1016/j.eurpolymj.2020.109946
Baari MJ, Megawati M, Benu DP. 1H and 13C NMR study of oligosuccinimide prepared by thermal condensation and evaluation of its scale inhibition. JKPK. 2022;7(3):287–302. doi:10.20961/jkpk.v7i3.65666
Cheng Y, Guo X, Zhao X, Wu Y, Cao Z, Cai Y, Xu Y. Nanosilica modified with polyaspartic acid as an industrial circulating water scale inhibitor. Clean Water. 2021;4(1):1–8. doi:10.1038/s41545-021-00137-y
Migahed MA, Rashwan SM, Kamel MM, Habib RE. Synthesized polyaspartic acid derivatives as corrosion and scale inhibitors in desalination operations. Cogent Eng. 2017;206(1):1–22. doi:10.1080/23311916.2017.1366255
Gao Y, Fan L, Ward L, Liu Z. Synthesis of polyaspartic acid derivative and evaluation of its corrosion and scale inhibition performance in seawater utilization. Desalination. 2015;365:220–226. doi:10.1016/j.desal.2015.03.006
Chen T, Zeng D, Zhou S. Study of polyaspartic acid and chitosan complex corrosion inhibition and mechanisms. Polish J Environ Stud. 2018;27(4):1441–1448. doi:10.15244/pjoes/78245
Cai YH, Zhao JL, Guo XY, Zhang XJ, Zhang RR, Ma SR, Cheng YM, Cao ZY, Xu Y. Synthesis of polyaspartic acid-capped 2-aminoethylamino acid as a green water treatment agent and study of its inhibition performance and mechanism for calcium scales. RSC Adv. 2022;12(38):24596–24606. doi:10.1039/D2RA04075A
Sangeetha Y, Meenakshi S, SairamSundaram, C. Corrosion mitigation of N-(2-hydroxy-3-trimethyl ammonium)propyl chitosan chloride as inhibitor on mild steel. Int J Biol Macromol. 2015;72:1244–1249. doi:10.1016/j.ijbiomac.2014.10.044
Chaudhari LP, Patel SN. Green approach to corrosion inhibition on mild steel in acidic media by the expired sulpha drug. Int J Manag Technol Eng. 2018;8(11):665–678. English. Available from: https://www.ijamtes.org/gallery/84.%20nov%20ijmte%20-%20as.pdf
Ahamad I, Prasad R, Quraishi MA. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros Sci. 2010;52(4):1472–1481. doi:10.1016/j.corsci.2010.01.015
Barmatov E, Hughes T, Nagl M. Efficiency of film-forming corrosion inhibitors in strong hydrochloric acid under laminar and turbulent flow conditions. Corros Sci. 2015;92:85–94. doi:10.1016/j.corsci.2014.11.038
Chen J, Xu L, Han J, Su M, Wu Q. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity. Desalination. 2015;358:42–48. doi:10.1016/j.desal.2014.11.010
Zhang YL, Zhao CX, Liu XD, Li W, Wang, JL, Hu ZG. Application of poly(aspartic acid-citric acid) copolymer compound inhibitor as an effective and environmental agent against calcium phosphate in cooling water systems. J Appl Res Technol. 2016;14(6):425–433. doi:10.1016/j.jart.2016.08.006
Yu W, Song D, Chen W, Yang H. Antiscalants in RO membrane scaling control. Water Res. 2020;183:115985. doi:10.1016/j.watres.2020.115985
Husna UZ, Elraies KA, Shuhili JABM, Elryes AA. A review:the utilization potency of biopolymer as eco-friendly scale inhibitors. J Pet Explor Prod Technol. 2022;12(4):1075–1094. doi:10.1007/s13202-021-01370-4
Huang H, Yao Q, Jiao Q, Liu B, Chen H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J Saudi Chem Soc. 2019;23(1):61–74. doi:10.1016/j.jscs.2018.04.003
Oshchepkov M, Golovesov V, Ryabova A, Tkachenko S, Redchuk A, Rönkkömäki H, Rudakova G, Pervov A, Popov K. Visualization of a novel fluorescent-tagged bisphosphonate behavior during reverse osmosis desalination of water with high sulfate content. Sep Purif Technol. 2021;255:117382. doi:10.1016/j.seppur.2020.117382
Ostolska I, Wiśniewska M. Comparison of the influence of polyaspartic acid and polylysine functional groups on the adsorption at the Cr2O3-Aqueous polymer solution interface. Appl Surf Sci. 2014;311:734-739. doi:10.1016/j.apsusc.2014.05.149
Guo X, Qiu F, Dong K, Zhou X, Qi J, Zhou Y, Yang D. Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan. J Ind Eng Chem. 2012;18(6):2177-2183. doi:10.1016/j.jiec.2012.06.015
Yang L, Li Y, Qian B, Hou B. Polyaspartic acid as a corrosion inhibitor for WE43 magnesium alloy. J Magnes Alloy. 2015;3(1):47–51. doi:10.1016/j.jma.2014.12.009
Duggirala P. Formation of calcium carbonate scale and control strategies in continuous digesters. CD del II Coloq. Int. sobre Celul [internet]. 2005. English. Available from: http://www.eucalyptus.com.br/icep02/prassad_duggirala
Kumar S, Naiya TK, Kumar T. Developments in oil field scale handling towards green technology—a review. J Petrol Sci Eng. 2018;169:42–444. doi:10.1016/j.petrol.2018.05.068
Hajirezaie S, Wu X, Reza M, Sakha S. Numerical simulation of mineral precipitation in hydrocarbon reservoirs and wellbores. Fuel. 2019;238:462–472. doi:10.1016/j.fuel.2018.10.101
Niedermayr A, Köhler SJ, Dietzel M. Impacts of aqueous carbonate accumulation rate, magnesium and polyaspartic acid on calcium carbonate formation (6–40 °C). Chem Geol. 2013;340:105–120. doi:10.1016/j.chemgeo.2012.12.014
de Souza FS, Spinelli A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros Sci. 2009;51(3):642–649. doi:10.1016/j.corsci.2008.12.013
Ma X, Jiang X, Xia S, Shan M, Li X, Yu L, Tang Q. New corrosion inhibitor acrylamide methyl ether for mild steel in 1M HCl. Appl Surf Sci. 2016;371:248–257. doi:10.1016/j.apsusc.2016.02.212
Hasson D, Shemer H, Sher A. State of the art of friendly ‘green’ scale control inhibitors:A review article. Ind Eng Chem Res. 2011;50(12):7601–7607. doi:10.1021/ie200370v
Mamand DM, Awla AH, Kak Anwer TM, Qadr HM. Quantum chemical study of heterocyclic organic compounds on the corrosion inhibition. Chim Techno Acta. 2022;9(2):1–11. doi:10.15826/chimtech.2022.9.2.03
Baari MJ, Pratiwi RY. Application of carbon dots as corrosion inhibitor:a systematic literature review. Indones J Chem. 2022;22(5):1427–1453. doi:10.22146/ijc.72327
Hadisaputra S, Hamdiani S, Kurniawan MA. Influence of macrocyclic ring size on the corrosion inhibition efficiency of dibenzo crown ether :a density functional study. Ind J Chem. 2017;17(3):431–438. doi:10.22146/ijc.26667
Radhi AH, Du EAB, Khazaal FA, Abbas ZM, Aljelawi OH, Hamadan SD, Almashhadani HA, Kadhim MM. HOMO-LUMO energies and geometrical structures effecton corrosion inhibition for organic compounds predict by DFT and PM3 methods. Neuro Quantol. 2020;18(1):37–45. doi:10.14704/nq.2020.18.1.NQ20105
Mishra A., Aslam J, Verma C, Quraishi MA, Ebenso EE. Imidazoles as highly effective heterocyclic corrosion inhibitors for metals and alloys in aqueous electrolytes: a review. J Taiwan Inst Chem Eng. 2020;114:341–358. doi:10.1016/j.jtice.2020.08.034
Ebenso EE, Isabirye DA, Eddy NO. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int J Mol Sci. 2010;11(6):2473–2498. doi:10.3390/ijms11062473
Fujimoto H, Inagaki S. Orbital interaction and chemical bonds. Polarization in chemical reactions. J Am Chem Soc. 1977; 99(23):7424–7432. doi:10.1021/ja00465a004
DOI: https://doi.org/10.15826/chimtech.2023.10.1.12
Copyright (c) 2023 Muhamad Jalil Baari

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







