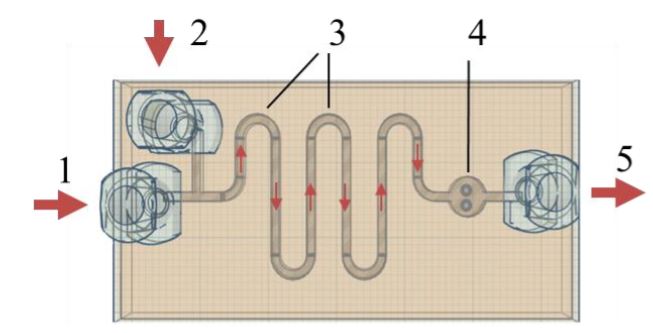
Portable potentiometric device for determining the antioxidant capacity
Abstract
Keywords
Full Text:
PDFReferences
Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: A review. Eur J Med Chem. 2019;178:687–704. doi:10.1016/j.ejmech.2019.06.010
Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55–74. doi:10.1016/j.ejmech.2015.04.040
Denisov ET, Afanas’ev IB. Oxidation and antioxidants in organic chemistry and biology. United Kingdom: Taylor & Francis Group; 2005. 992 p.
Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical report). Pure Appl Chem. 2013:85(5):957–998. doi:10.1351/PAC-REP-12-07-15
Arts M JT J, Dallinga JS, Voss H-P, Haenen GRMM, Bast A. A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chem. 2003;80(3):409–414. doi:10.1016/С0308-8146(02)00468-5
Van Den Berg R, Haenen GRMM, Van Den Berg H, Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66(4):511–517. doi:10.1016/S0308-8146(99)00089-8
Wayner DDM, Burton GW, Ingold KU, Barclay LRC, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta 1987;924(3):408–419. doi:10.1016/0304-4165(87)90155-3
Wayner DDM. Radical-trapping antioxidants in vitro and in vivo. Bioelectrochem Bioenerg. 1987;18(1–3):219–229. doi:10.1016/0302-4598(87)85024-9
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Deemer EK. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J Agric Food Chem. 2002;50(7):1815–1821. doi:10.1021/jf0113732
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: the FRAP assay. Anal Biochem. 1996;239:70‒76. doi:10.1006/abio.1996.0292
Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektaşoğlu B, Berker KI, Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molec. 2007;12:1496‒1547. doi:10.3390/12071496
Benera M, Apak R. Ferric-o-phenanthroline adsorbed on a Nafion membrane: A novel optical sensor for antioxidant capacity measurement of food extracts. Sens Actuators B Chem. 2017;247:155–162. doi:10.1016/j.snb.2017.03.017
Bener M, Ozyurek M, Guclu K, Apak R. Development of a low-cost optical sensor for cupric reducing antioxidant capacity measurement of food extracts. Anal Chem. 2010;82:4252–4258. doi:10.1021/ac100646k
Apak R, Guclu K, Ozyurek M, Karademir S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J Agric Food Chem. 2004;52:7970−7981. doi:10.1021/jf048741x
Sharpe E, Frasco T, Andreescu D, Andreescu S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Anal. 2013;138:249–262. doi:10.1039/c2an36205h
Arikana B, Alpa FN, Ozfidan-Konakcib C, Balcia M, Elbasana F, Yildiztugaya E, Cavusoglu H. Fe2O3-modified graphene oxide mitigates nanoplastic toxicity via regulating gas exchange, photosynthesis, and antioxidant system in Triticum aestivum. Chemosphere. 2022;307(4):136048. doi:10.1016/j.chemosphere.2022.136048
Zhanga S, Chena J, Lianb M-L, Yanga W-Sh, Chena X. An engineered, self-propelled nanozyme as reactive oxygen species scavenger. Chem Engineer J. 2022;446(2):136794. doi:10.1016/j.cej.2022.136794
Ivanova A, Gerasimova E, Gazizullina E, Borisova M, Drokin R, Gorbunov E, Ulomskiy E, Rusinov V. The antioxidant screening of potential materials for drugs based on 6-nitro-1,2,4-triazoloazines containing natural polyphenol fragments. Anal Bioanal Chem. 2020;412:5147–5155. doi:10.1007/s00216-020-02466-2
Ivanova A, Gerasimova E, Gazizullina E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molec. 2020;25:4251. doi:10.3390/molecules25184251
Özbeka O, Berkel C. Recent advances in potentiometric analysis: Paper–based devices. Sens Intern. 2022;3:100189. doi:10.1016/j.sintl.2022.100189
Özbek O, Isildak Ö. Potentiometric determination of copper(II) ions based on a porphyrin derivative. J Chin Chem Soc. 2022;69(7):1060–1069. doi:10.1002/jccs.202200168
Özbek O, Isildak Ö. Potentiometric PVC membrane sensor for the determination of anti-epileptic drug levetiracetam in Pharmaceutical formulations. Chem Sel. 2022;7(3):1–8. doi:10.1002/slct.202103988
Özbek O, Isildak Ö. Use of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin as sensor material: potentiometric determination of aluminium(III) ions. Bull Mater Sci. 2022;45(114):1–10. doi:10.1007/s12034-022-02696-3
Ivanova AV, Gerasimova EL, Brainina KhZ. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit Rev Anal Chem. 2015;45:311–322. doi:10.1080/10408347.2014.910443
Ivanova AV, Gerasimova EL, Gazizullina ER. An integrated approach to the investigation of antioxidant properties by potentiometry. Anal Chim Acta. 2020;111:83–91. doi:10.1016/j.aca.2020.03.041
Eksperiandova LP, Belikov KN, Khimchenko SV, Blank TA. Once again about determination and detection limits. J Anal Chem. 2010;65(3):223–228. doi:10.1134/S1061934810030020
Shpigun LK, Arharova MA, Brainina KhZ, Ivanova AV. Flow injection potentiometric determination of total antioxidant activity of plant extracts. Anal Chim Acta. 2006;573(574):419–426. doi:10.1016/j.aca.2006.03.094
Brainina KhZ, Alyoshina LV, Gerasimova EL, Kazakov YaE, Beykin YaB, Belyaeva SV, Usatova TI, Inzhevatova OV, Ivanova AV, Khodos MY. New electrochemical methods of determining antioxidant activity of blood and blood fractions. Electroanal. 2009;21:618–624. doi:10.1002/elan.200804458
Menshchikov EB, Lankin VZ, Kandalintseva NV. Phenolic antioxidants in biology and medicine. Structures, properties, mechanisms of action. Germany: LAP LAMBERT Academic Publishing; 2012. 488 p.
Padayatty SJ, Katz A, Wang Ya, Eck P, Kwon O, Lee Je-H, Chen Sh, Corpe Ch, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):1835. doi:10.1080/07315724.2003.10719272
Bensalah N, Trabelsi H, Gadri A. Electrochemical treatment of aqueous wastes containing pyrogallol by BDD-anodic oxidation. J Environ Manage. 2009;90:523–530. doi:10.1016/j.jenvman.2007.12.007
Zinatullina KM, Kasaikina OT, Kuzmin VA, Khrameeva NP. Pro- and antioxidant characteristics of natural thiols. Rus Chem Bull. 2018;67(4):726‒730. doi:10.1007/s11172-018-2129-0
Anderson ME. Glutathione and glutathione delivery compounds. Adv Pharmacol. 1996;38:65‒78. doi:10.1016/S1054-3589(08)60979-5
Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi:10.1007/s12013-009-9043-х
Ivanova A, Gerasimova E, Gazizullina E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molec. 2020;25:4251. doi:10.3390/molecules25184251
Buck R P, Lindner E. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994). Pure Appl Chem. 1994;66(12):2527–2536. doi:10.1351/pac199466122527
DOI: https://doi.org/10.15826/chimtech.2023.10.1.04
Copyright (c) 2022 Elena R. Salimgareeva, Dinara I. Igdisanova, Daria S. Gordeeva, Anatoly I. Matern, Elena. A. Yarkova, Elena L. Gerasimova, Alla V. Ivanova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







