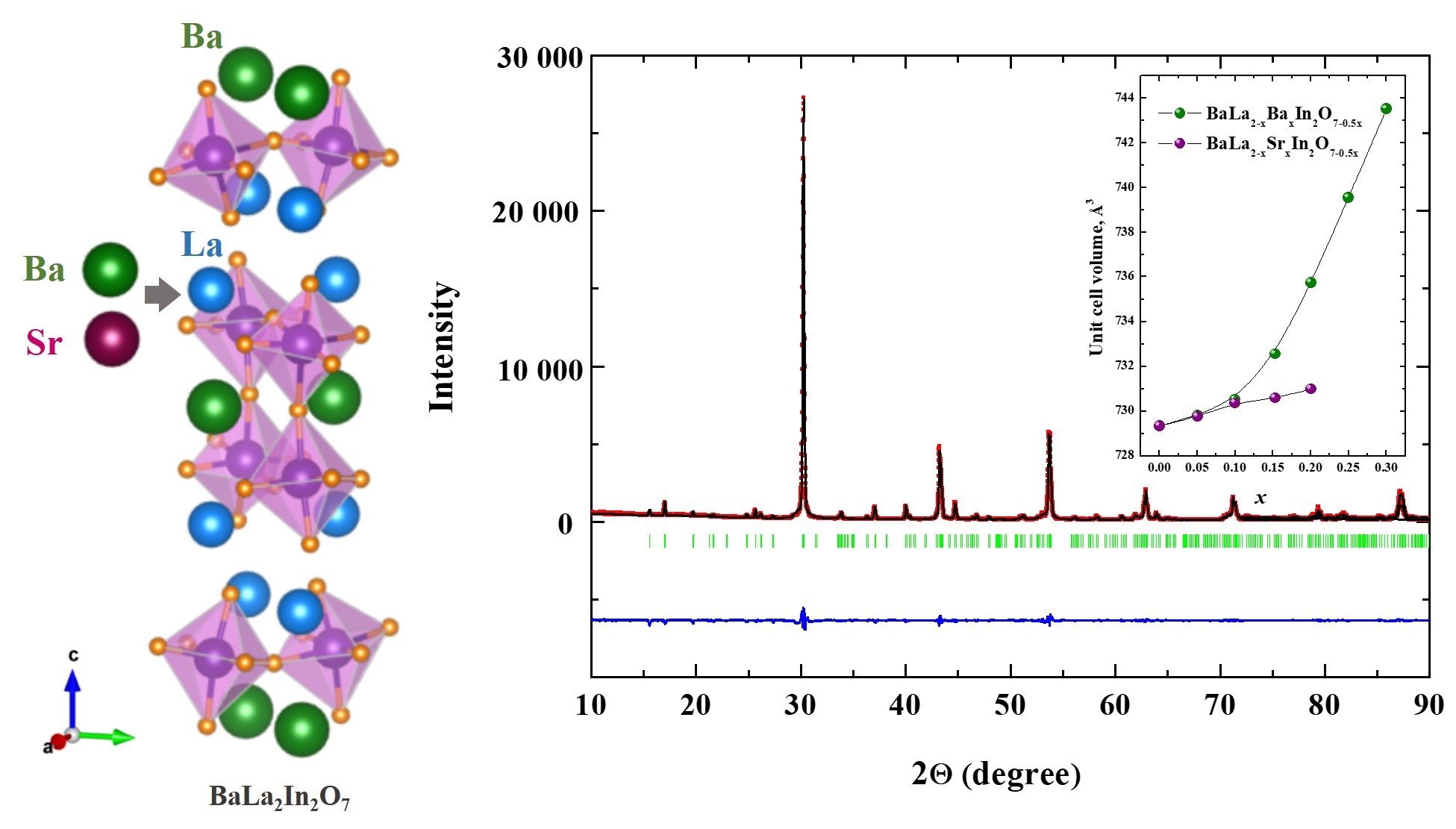
Local structure and ionic transport in acceptor-doped layered perovskite BaLa2In2O7
Abstract
Keywords
Full Text:
PDFReferences
Punj P, Singh J, Singh K. Ceramic biomaterials: Properties, state of the art and future prospectives. Ceram Int. 2021;47:28059–28074. doi:10.1016/j.ceramint.2021.06.238
Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nature Rev Mater. 2020;5:584–603. doi:10.1038/s41578-020-0204-2
Weng W, Wu W, Hou M, Liu T, Wang T, Yang H. Review of zirconia-based biomimetic scaffolds for bone tissue engi-neering. J Mater Sci. 2021;56:8309–8333. doi:10.1007/s10853-021-05824-2
Rosso JM, Volnistem EA, Santos IA, Bonadio TGM, Freitas VF. Lead-free NaNbO3-based ferroelectric perovskites and their polar polymer-ceramic composites: Fundamentals and potentials for electronic and biomedical applications. Ceram Int. 2022;48:19527-19541. doi:10.1016/j.ceramint.2022.04.089
Tarasova N, Galisheva A, Belova K, Mushnikova A, Volo-kitina E. Ceramic materials based on lanthanum zirconate for the bone augmentation purposes: materials science approach. Chimi Techn Acta. 2022;9:20229209. doi:10.15826/chimtech.2022.9.2.09
Duan C, Huang J, Sullivan N, O'Hayre R. Proton-conducting oxides for energy conversion and storage. Appl Phys Rev. 2022;7:011314. doi:10.1063/1.5135319
Abdalla AM, Hossain S, Nisfindy OB, Azad AT, Dawood M, Azad AK. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers Manag. 2018;165:602–627. doi:10.1016/j.enconman.2018.03.088
Kim J, Sengodan S, Kim S, Kwon O, Bu Y, Kim G. Proton con-ducting oxides: A review of materials and applications for renewable energy conversion and storage. Renewable and Sustainable. Energy Rev. 2019;109:606–618. doi:10.1016/j.rser.2019.04.042
Medvedev DA, Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr Opin Green Sustain Chem. 2021;32:100549. doi:10.1016/j.cogsc.2021.100549
Zvonareva I., Fu XZ, Medvedev D, Shao Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2021;15:439-465. doi:10.1039/D1EE03109K
Ruddlesden SN, Popper P. New compounds of the K2NiF4 type. Acta Cryst. 1957;10:538–539. doi:10.1107/S0365110X57001929
Ruddlesden SN, Popper P. The compound Sr3Ti2O7 and its structure. Acta Cryst. 1958;11:54–55. doi:10.1107/S0365110X58000128
Aurivillius B. Mixed Bismuth Oxides with Layer Lattices: I. Structure Type of CaBi2B2O9. Arkiv Kemi. 1949;1:463–480.
Dion M, Ganne M, Tournoux M. Nouvelles familles de phases MIMII2Nb3O10 a feuillets «perovskites». Mat Res Bull. 1981;16:1429–1435.
Jacobson AJ, Lewandowski JT, Johnson JW Ion exchange of the layered perovskite KCa2Nb3O10 by protons. J Less-Common Metal. 1986;116:137–145.
Jacobson AJ, Lewandowski JT, Johnson JW. Interlayer chemistry between thick transition-metal oxide layers: synthesis and intercalation reactions of K[Ca2Nan-3NbnO3n+1]. Inorg Chem. 1985;24:3727–3729.
Rodionov IA, Zvereva IA. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ Chem Rev. 2016;85248–85279. doi:10.1070/RCR4547
Krasheninnikova OV, Syrov EV, Smirnov SM, Suleimanov EV, Fukina DG, Knyazev AV, Titaev DN. Synthesis, crystal structure and photocatalytic activity of new Dion-Jacobson type titanoniobates. J Solid State Chem. 2022;315:123445. doi:10.1016/j.jssc.2022.123445
Chawla H, Chandra A, Ingole P P, Garg S. Recent advance-ments in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. J Ind Eng Chem. 2021;95:1–15. doi:10.1016/j.jiec.2020.12.028
Ferreira WC, Rodrigues GLC, Araújo BS, de Aguiar FAA, de Abreu Silva, ANA, Fechine, PBA, de Araujo Paschoal CW; Ayala AP. Pressure-induced structural phase transitions in the multiferroic four-layer Aurivillius ceramic Bi5FeTi3O15. Ceram Int. 2020; 46:18056–180621. doi:10.1016/j.ceramint.2020.04.122
Zulhadjri Wendari TP, Ikhram M, Putri YE, Septiani U, Imelda. Enhanced dielectric and ferroelectric responses in La3+/Ti4+ co-substituted SrBi2Ta2O9 Aurivillius phase. Ceram Int. 2022; 48:10328–103321. doi:10.1016/j.ceramint.2022.01.307
Xu Q, Xie S, Wang F, Liu J, Shi J, Xing J, Chen Q, Zhu J, Wang Q. Bismuth titanate based piezoceramics: Structural evolutions and electrical behaviors at different sintering temperatures. J Alloys Compd. 2021;88215:160637. doi:10.1016/j.jallcom.2021.160637
Mamidi S, Gundeboina R, Kurra S, Velchuri R, Muga V. Au-rivillius family of layered perovskites, BiREWO6 (RE = La, Pr, Gd, and Dy): Synthesis, characterization, and photocatalytic studies. Comptes Rendus Chimie. 2018;21:547–552. doi:10.1016/j.crci.2018.01.011
Zhou G, Jiang X, Zhao J, Molokeev M, Lin Z, Liu Q, Xia Z. Two-Dimensional-Layered Perovskite ALaTa2O7:Bi3+ (A = K and Na) phosphors with versatile structures and tunable photoluminescence. ACS Appl Mater Interfaces. 2018;10:24648–2465525. doi:10.1021/acsami.8b08129
Panda DP, Singh AK, Kundu TK, Sundaresan A. Visible-light excited polar Dion-Jacobson Rb(Bi1-xEux)2Ti2NbO10 perovskites: Photoluminescence properties and in vitro bioimag-ing. J Mater Chem. B 2022;10:935–944. doi:10.1039/d1tb02445k
Tarasova N, Animitsa I. AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Mater. 2022;15(1):114. doi:10.3390/ma15010114
Ishigaki T, Hester JR. New perovskite-related structure family of oxide-ion conducting Materials NdBaInO4. Chem Mater. 2014;26(8):2488–2491. doi:10.1021/cm500776x
Fujii K, Shiraiwa M, Esaki Y, Yashima M, Kim SJ, Lee S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J Mater Chem A. 2015;3(22):11985–11990. doi:10.1039/c5ta01336d
Ishihara T, Yan Y, Sakai T, Ida S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016;288:262–265. doi:10.1016/j.ssi.2016.01.011
Yang X, Liu S, Lu F, Xu J, Kuang X. Acceptor Doping and oxygen vacancy migration in layered perovskite NdBaInO4 based mixed conductors. J Phys Chem C. 2016;12:6416–6426. doi:10.1021/acs.jpcc.6b00700
Fujii K, Yashima M. Discovery and development of BaNdInO4 – A brief review. J Ceram Soc Japan. 2018;126(10):852–859. doi:10.2109/jcersj2.18110
Zhou Y, Shiraiwa M, Nagao M, Fujii K, Tanaka I, Yashima M, Baque L, Basbus JF, Mogni LV, Skinner SJ. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem Mater. 2021;33(6):2139–2146. doi:10.1021/acs.chemmater.0c04828
Kato S, Ogasawara M, Sugai M, Nakata S. Synthesis and oxide ion conductivity of new layered perovskite La1-xSr1+xInO4-d. Solid State Ion. 2002;149(1–2):53–57.doi:10.1016/S0167-2738(02)00138-8
Troncoso L, Alonso JA, Aguadero A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1-xInO4+d. J Mater Chem A. 2015;3(34):17797–17803. doi:10.1039/c5ta03185k
Troncoso L, Alonso JA, Fernández-Díaz MT, Aguadero A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xB¬xO4+δ system (B=Zr, Ti). Solid State Ion. 2015;282:82–87. doi:10.1016/j.ssi.2015.09.014
Troncoso L, Mariño C, Arce MD, Alonso JA. Dual oxygen defects in layered La1.2Sr0.8-xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: a neutron diffraction study. Mater. 2019;12(10):1624. doi:10.3390/ma12101624
Troncoso L, Arce MD, Fernández-Díaz MT, Mogni LV, Alonso JA. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8-xBaxInO4+d. New J Chem. 2019;43(15):6087–6094. doi:10.1039/C8NJ05320K
Shiraiwa M, Kido T, Fujii K, Yashima M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J Mat Chem A. 2021;9:8607. doi:10.1039/D0TA11573H
Tarasova N, Animitsa I, Galisheva A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Comm. 2021;323:114093. doi:10.1016/j.ssc.2020.114093
Tarasova N, Galisheva A, Animitsa I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics. 2020;26:5075–5088. doi:10.1007/s11581-020-03659-6
Tarasova N, Galisheva A, Animitsa I. Ba2+/Ti4+- co-doped layered perovskite BаLaInO4: the structure and ionic (O2−, H+) conductivity. Int J Hydrog Energy. 2021;46(32):16868–16877. doi:10.1016/j.ijhydene.2021.02.044
Tarasova N, Galisheva A, Animitsa I, Anokhina I, Gilev P, Cheremisina P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram Int. 2022;48(11):15677–15685. doi:10.1016/j.ceramint.2022.02.102
Tarasova N, Galisheva A, Animitsa I, Korona D, Davletbaev K. Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: Synthesis, hydration, ionic (O2+, H+) conductivity. Int J Hydrog Energy. 2022;47(44):18972–18982. doi:10.1016/j.ijhydene.2022.04.112
Tarasova N, Bedarkova A. Advanced proton-conducting ceramics based on layered perovskite BaLaInO4 for energy conversion technologies and devices. Mater. 2022;15:6841. doi:10.3390/ma15196841
Tarasova N, Galisheva A, Animitsa I, Korona D, Kreimesh H, Fedorova I. Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl Sci. 2022;12(8):4082. doi:10.3390/app12084082
Tarasova N, Bedarkova A, Belova K, Abakumova E, Cheremisina P, Medvedev D. Oxygen ion and proton transport in alkali-earth doped layered perovskites based on BaLa2In2O7. Inorg. 2022;10:161. doi:10.3390/inorganics10100161
Tarasova N, Galisheva A, Animitsa I, Belova K, Egorova A, Abakumova E, Medvedev D. Layered Perovskites BaM2In2O7 (M = La, Nd): from the structure to the ionic (O2–, H+) conductivity. Mater. 2022;15:3488. doi:10.3390/ma15103488
Tarasova N, Galisheva A. Phosphorus-doped protonic con-ductors based on BaLanInnO3n+1 (n = 1, 2): applying oxyanion doping strategy to the layered perovskite structures. Chim Tehno Acta 2022;9:20229405. doi:10.15826/chimtech.2022.9.4.05
Tarasova N, Animitsa I, Galisheva A, Spectroscopic and transport properties of Ba- and Ti-doped BaLaInO4. J Raman Spec. 2021;52:980–987. doi:10.1002/jrs.6078
Tarasova N, Animitsa I, Galisheva A, Effect of doping on the local structure of new block-layered proton conductors based on BaLaInO4. J Raman Spec. 2021;51:2290–2297. doi:10.1002/jrs.5966
Shannon RD, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–767. doi:10.1107/S0567739476001551
Scherban T, Villeneuve R, Abello L, Lucazeau G. Raman scattering study of acceptor-doped BaCeO3. Solid State Ion. 1993;61:93–98. doi:10.1016/0167-2738(93)90339-5
Chemarin C, Rosman N, Pagnier T, Lucazeau G. High-pressure raman study of mixed perovskites BaCexZr1-xO3 (0≤x≤1). J Solid State Chem. 2000;149:298–307. doi:10.1006/jssc.1999.8530
Kamba S, SamoukPina F, Kadlec F, Pokorny J, Petzelt J, Rea-ney IM, Wise PL. Composition dependence of the lattice vibrations in Srn+1TinO3n+1 Ruddlesden–Popper homologous series. J Eur Ceram Soc. 2003;23:2639–2645. doi:10.1016/S0955-2219(03)00150-X
Iliev MN, Popov VN, Litvinchuk AP, Abrashev MV, Backstrom J, Sun YY, Mena RL, Chu CW. Comparative Raman studies of Sr2RuO4; Sr3Ru2O7 and Sr4Ru3O10. Phys B. 2005;358:138–152. doi:10.1016/j.physb.2004.12.069
DOI: https://doi.org/10.15826/chimtech.2022.9.4.15
Copyright (c) 2022 Nataliia Tarasova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







