Polymer-metal complex based on copper(II) acetate and polyvinyl alcohol: thermodynamic and catalytic properties
Abstract
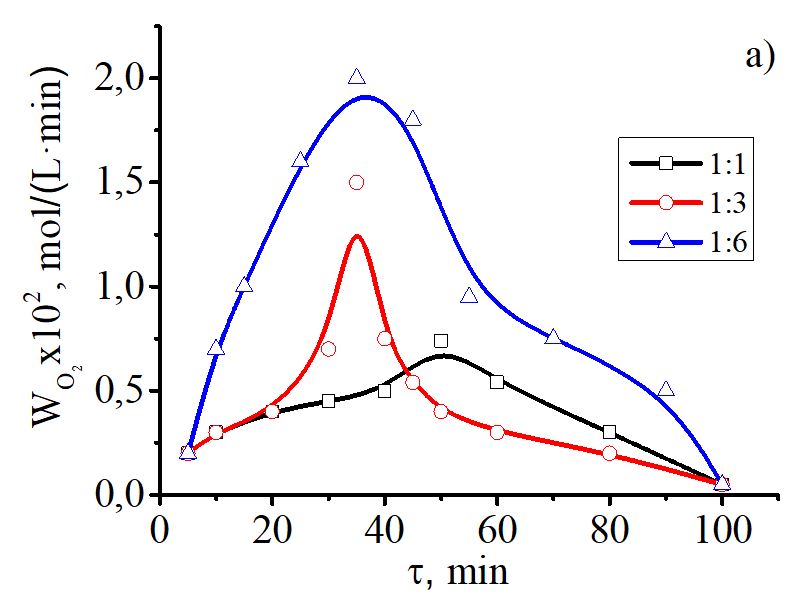
In this work we obtained a polymer-metal complex by mixing aqueous solution of copper(II) acetate with PVA at a certain ratio, pH of the solution and temperature. The composition of the complex compound was determined by potentiometric and conductometric titration. The possibility of a complex formation was proved by calculating thermodynamic characteristics. The stability constant of the polymer-metal complex was calculated on the basis of the modified Bjerrum’s method. The metal-polymer complex was synthesized in the ratio 1:2. IR spectroscopy and scanning electron microscopy (SEM) confirmed the coordination of polymeric PVA ligand to copper and allowed evaluating the morphology and features of the complex surface. The catalytic activity of the synthesized compound was evaluated in the oxidation reaction of elemental phosphorus (P4) by oxygen in aqueous-organic media under mild conditions. Quantitative analysis of phosphoric acid was made by photocolorimetric method. We found that the oxidation process of P4 in the presence of the complex Cu(PVA)2(OAc)2 in aqueous-organic media is characterized with the maximum absorption rate, in comparison with Cu(OAc)2·H2O oxidation process with P4, and yields up to 97% of the products. The process of oxidation of yellow phosphorus by oxygen in the presence of the copper(II)-PVA complex proceeds through key reactions of two-electron reduction of the catalyst P4 with the formation of intermediate phosphorus-containing products P3+ and the stages of catalyst regeneration by oxygen. Twenty-electron oxidation of P4 to the phosphorus-containing P5+ products involves 10 two-electron redox reactions and a number of complexation or hydrolysis stages.
Keywords
Full Text:
PDFReferences
Sorokin AB, Kudrik EV. Phthalocyanine metal com-plexes: versatile catalysts for selective oxidation and bleaching. Catalysis Today. 2017;159:37–46. doi:10.1016/j.cattod.2010.06.020
Gunasekera SP, Kashman Y, Cross SS, Lui MS, Pomponi SA, Diaz MС. Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: antiviral and antitumor bis(indolyl)imidazoles from caribbean deep-sea sponges of the family halichondriidae. Structural and synthetic studies. J Org Chem. 1988;53(23):5446–5453. doi:10.1021/jo00258a009
Goyal R, Singh O, Agrawal A, Samanta C, Sarkar B. Advantages and limitations of catalytic oxidation with hydrogen peroxide: from bulk chemicals to lab scale process. Catal Rev. 2020;64(2):1–57. doi:10.1080/01614940.2020.1796190
Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit Rev Environ Sci Technol. 2006;36:1–84. doi:10.1080/10643380500326564
Hassaan MA, Nemr AEl, El-Zahhar AA, Idris AM, Alghamdi MM, Sahlabji T, Said TO. Degradation mechanism of direct red 23 dye by advanced oxidation processes: a comparative study. Toxin Rev. 2020;41(1):1–10. doi:10.1080/15569543.2020.1827431
Topalovic T, Nierstrasz V, Bautista L, Jocic D, Navarro R, Warmoeskerken M. Analysis of the effects of catalytic bleaching on cotton. Cellulose. 2017;14:385–400. doi:10.1007/s10570-007-9120-5
Das L, Kolar P, Sharma-Shivappa R. Heterogeneous catalytic oxidation of lignin into value-added chemicals. Biofuels. 2012;3(2):155–166. doi:10.4155/bfs.12.5
Singh K, Arora S. Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit Rev Environ Sci Technol. 2011;41:807–878. doi:10.1080/10643380903218376
Shindhal T, Rakholiya P, Varjani S, Pandey A, Ngo HH, Guo W, Ng HY, Taherzadeh MJ. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioeng. 2020;12(1):70–87. doi:10.1080/21655979.2020.1863034
Crabtree HR. The Organometallic Chemistry of the Transition Metals. 5th ed. New Jersey: John Wiley & Sons, Inc.; 2005. 496 p. doi:10.1002/9781118788301
Spessard UO, Miessler UL. Organometallic Chemistry. 3rd ed. Oxford: Oxford University Press; 2015. 800 p.
Wang Y, Wang M, Wang L, Wang Y, Wang X, Sun L. Asymmetric oxidation of sulfides with H2O2 catalyzed by titanium complexes of Schiff bases bearing a dicumenyl salicylidenyl unit. Appl Organometal Chem. 2011;25:325–330. doi:10.1002/aoc.1762
Papastergiou M, Stathi P, Milaeva ER, Deligiannakis Y, Louloudi M. Comparative study of the catalytic thermodynamic barriers for twohomologous Mn- and Fe-non-heme oxidation catalysts. J Catal. 2016;341:104–115. doi:10.1016/j.jcat.2016.06.017
Zhou B, Zhang Zh, Li Y, Han G, Feng Y, Wang B, Zhang D, Ma J, Liu C. Flexible, Robust, and Multifunctional Electromagnetic Interference Shielding Film with Alternating Cellulose Nanofiber and MXene Layers. ACS Appl Mater Interfaces. 2020;12(4):4895–4905. doi:10.1021/acsami.9b19768
Mukul B, Subrata M. Synthesis and some properties of PVC bonded complexes. J Appl Polym Sci. 1989;33:1243. doi:10.1002/app.1989.070380705
James SL. Metal-organic frameworks. Chem Soc Rev. 2003;32:276–288. doi:10.1039/B200393G
Janiak C. Engineering coordination polymers towards applications. Dalton Trans. 2003;14:2781–2804. doi:10.1039/B305705B
Maspoch D, Ruiz-Molina D, Veciana J. Magnetic nanoporous coordination polymers. J Mater Chem. 2004; 14:2713-2723. doi:10.1039/B407169G
Batten SR, Murray KS. Structure and magnetism of coordination polymers containing dicyanamide and tricy-anomethanide. Coord Chem Rev. 2003;246(1–2):103–130. doi:10.1016/S0010-8545(03)00119-X
Annas Al-Sharabi, Abdullah M Al-Hussam, Sami KS Abdullh. Synthesis and characterization of metal complexes of Cu(II) and Cd(II) with polyvinyl alcohol and stadied of electrical and optical properties. Int J Multidis Res Dev. 2019;6(12):19–25. Available from: https://www.allsubjectjournal.com/archives/2019/vol6/issue12/6-11-37
Rajendran S, Mahendran O. Experimental investigations on plasticized PMMA/PVA polymer blend electrolytes. Ionics. 2001;7:463–468. doi:10.1007/BF02373585
Postnikov IN. Thermal phosphoric acid, salts and fertilizers on its basis. Chemia: Moscow; 1980. 330 p.
Akbayeva DN, Bakirova BS, Seilkhanova GA, Sitzmann H. Oxidation of octene-1 in the presence of palladium-polyvinylpyrrolidone complex. Bull Chem Reac Eng Cat. 2018;3:560–572. doi:10.9767/bcrec.13.3.1980.560-572
Hudson R. Structure and Mechanism of Reactions of Organophosphorus Compounds. Mir: Moscow; 1967. 357 p.
Scheer M, Becker U, Magull J. The reaction of P4 with [Cp’Mo(CO)3]2 (Cp’ = 5-C5H4t-Bu) – the structure of Cp’Mo(CO)2(3-P4){Cr(CO)54(H). Polyhedron. 1998;17(11–12):1983–1989. doi:10.1016/S0277-5387(97)00480-4
Scheer M. Metal element triple bonds of the heavier group 15 elements. Coord Chem Rev. 1997;163:271–286. doi:10.1016/S0010-8545(97)00014-3
DOI: https://doi.org/10.15826/chimtech.2022.9.3.04
Copyright (c) 2022 Kuralay S. Maksotova, Dariya Т. Kalikh, Arnur T. Omirzakova, Botagoz S. Bakirova, Dina N. Akbayeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







