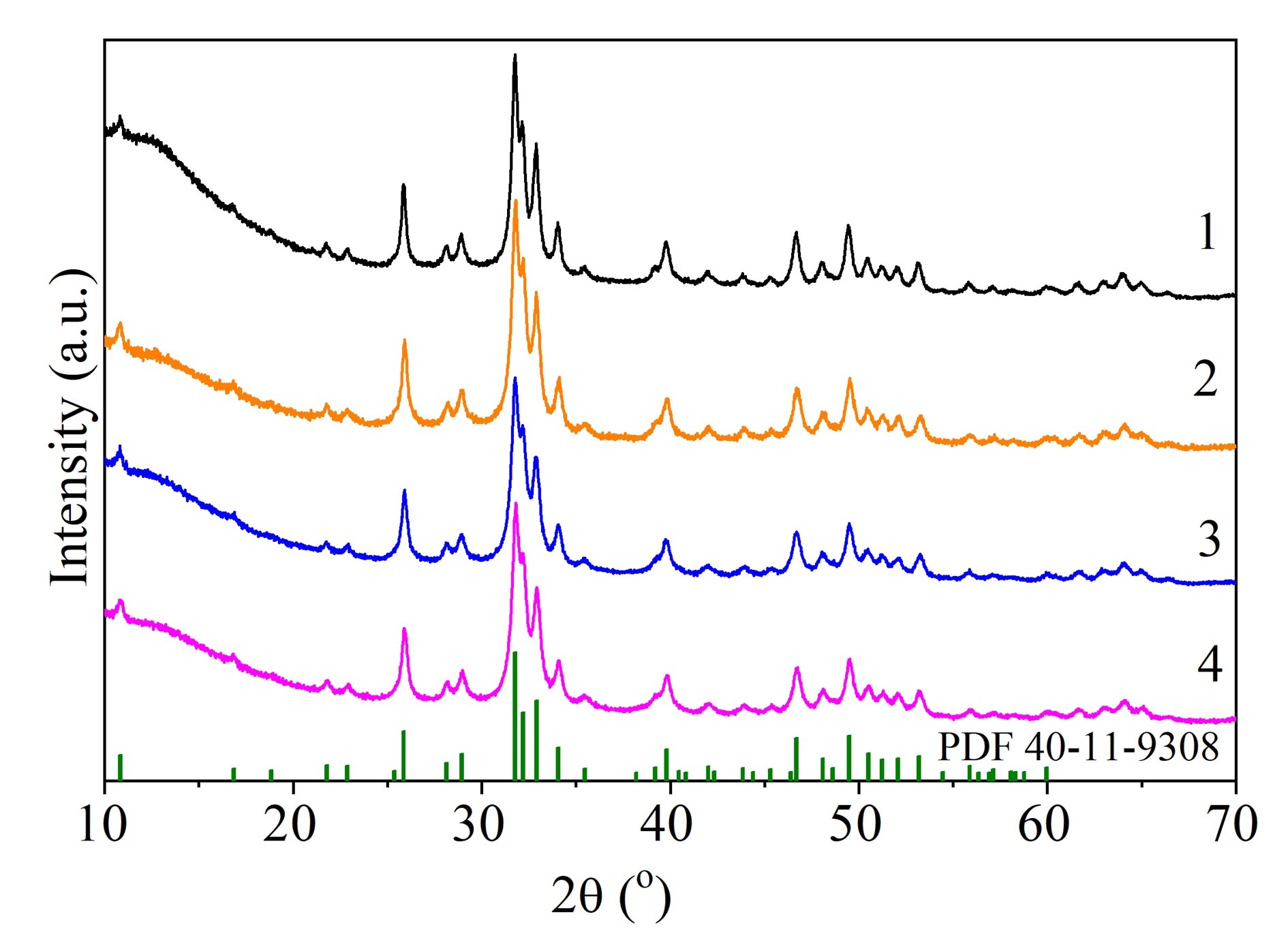
Soft mechanochemical synthesis and thermal stability of hydroxyapatites with different types of substitution
Abstract
Keywords
Full Text:
PDFReferences
Hughes M, Rakovan J. The crystal structure of apatite, Ca5(PO4)3(F,OH,Cl)2. Rev Mineral Geochem. 2002:48(1):1–12. doi:10.2138/rmg.2002.48.1
Dorozhkin SV. Calcium orthophospates (CaPO4): occurrence and properties. Prog Biomater. 2016:5(1):9–70. doi:10.1007/s40204-015-0045-z
Mucalo MR. Hydroxyapatite (HAp) for biomedical applications: Woodhead Publishing Limited Waltham; 2015. 364 p.
Kenny SM, Buggy M. Bone cements and fillers: a review. J Mater Sci Mater Med. 2003:14(11):923–938. doi:10.1023/A:1026394530192
Kumar A, Kargozar S, Baino F, Han S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds. A Rev Front Mater. 2019:6:313. doi:10.3389/fmats.2019.00313
Han Y, Wei Q, Chang P, Hu K, Okoro OV, Shavandi A, Nie L. Three-dimensional printing of hydroxyapatite composites for biomedical application. Cryst. 2021:11(4):353. doi:10.3390/cryst11040353
Mondal S, Dorozhkin SV, Pal U. Mondal S, Dorozhkin SV, Pal U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. WIREs Nanomed Nanobiotechnol. 2018:10(4):e1504. doi:10.1002/wnan.1504
Šupová M. Substituted hydroxyapatites for biomedical applications: a review. Ceram Int. 2015:41(8):9203–9231. doi:10.1016/j.ceramint.2015.03.316
Dorozhkin SV. Calcium orthophosphates in nature, biology and medicine. Mater. 2009:2(2): 399–498. doi:10.3390/ma2020399
Kolmas J, Groszyk E, Kwiatkowska-Różycka D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Res Int. 2014;2014:178123. doi:10.1155/2014/178123
Patel N, Best SM, Bonifield W, Gibson IR, Hing KA, Damien E, Revell PA. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002:13(12):1199–1206. doi:10.1023/a:1021114710076
Anwar A, Akbar S, Sadiqa A, Kazmi M. Novel continuous flow synthesis, characterization and antibacterial studies of nanoscale zinc substituted hydroxyapatite bioceramics. Inorg Chim Acta. 2016:453:16–22. doi:10.1016/j.ica.2016.07.041
Mondal S, Manivasagan P, Bharathiraja S, Moorthy MS, Kim HH, Seo H, Lee KD, Oh J. Magnetic hydroxyapatite: a promising multifunctional platform for nanomedicine application. Int J Nanomed. 2017:12:8389–8410. doi:10.2147/IJN.S147355
Bulina NV, Chaikina MV, Prosanov IY, Gerasimov KB, Ishchenco AV, Dudina DV. Mechanochemical synthesis of SiO44– - substituted hydroxyapatite, part III – thermal stability. Eur J Inorg Chem. 2016:2016(12):1866–1874. doi:10.1002/ejic.201501486
Othmani M, Bachoua H, Ghandour Y, Aissa A, Debbabi M. Synthesis, characterization and catalytic properties of copper-substituted hydroxyapatite nanocrystals. Mater Res Bull. 2018:97:560–566. doi:10.1016/j.materresbull.2017.09.056
Chaikina MV, Bulina NV, Vinokurova OB, Prosanov IYu, Dudina DV. Interaction of calcium phosphates with calcium oxide or calcium hydroxide during the “soft” mechanochemical synthesis of hydroxyapatite. Ceram Int. 2019:45(14):16927–16933. doi:10.1016/j.ceramint.2019.05.239
Makarova SV, Bulina NV, Prosanov IY, Chaikina MV, Ishcenko AV. Mechanochemical synthesis of apatite with the simultaneous substitutions of calcium by lanthanum and phosphate by silicate. Russ J Inorg Chem. 2020:65(12):1831-1837. doi:10.1134/S0036023620120116
Baikie T, Ng GM, Madhavi S, Pramana SS, Blake K, Elcombe M, White TJ. The crystal chemistry of the alkaline-earth apatites A10(PO4)6CuxOy(H)z (A = Ca, Sr and Ba). Dalton Trans. 2009:34: 6722–6726. doi:10.1039/b906639j
Gomes S, Kaur A, Nedelec JM, Renaudin G. X-ray absorption spectroscopy shining (synchrotron) light onto the insertion of Zn2+ in calcium phosphate ceramics and its influence on their behavior under biological conditions. J Mater Chem B. 2014:2(5):536–545. doi:10.1039/C3TB21397H
Karpov AS, Nuss J, Jansen M, Kazin PE, Tretyakov YD. Synthesis, crystal structure and properties of calcium and barium hydroxyapatites containing copper ions in hexagonal channels. Solid State Sci. 2003:5(9):1277–1283. doi:10.1016/S1293-2558(03)00152-3
DOI: https://doi.org/10.15826/chimtech.2022.9.3.05
Copyright (c) 2022 Natalya V. Eremina, Svetlana V. Makarova, Denis D. Isaev, Natalya V. Bulina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







