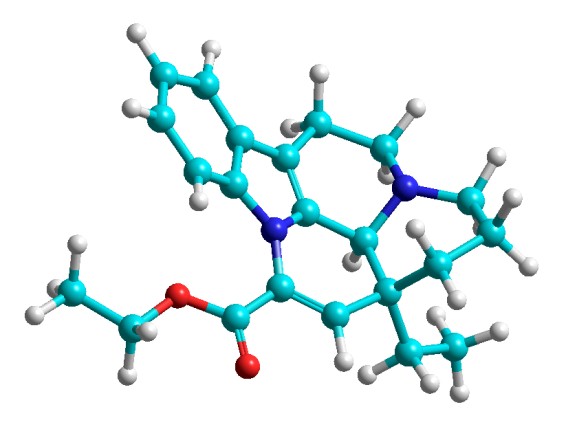
PEG- 4000 increases solubility and dissolution rate of vinpocetin in solid dispersion system
Abstract
In the present work investigations were performed concerned with the determination of the optimal ratio of vinpocetine and polyethylene glycol within a solid dispersion (1:2 or 1:5) according to the simulation results in the framework of molecular dynamics associated with the release of the reactant into aqueous medium. For the simulation of vinpocetine release from its alloy with polyethylene glycol a technique of coarse-grained molecular dynamics in the force field of Martini 2.2 was applied with the use of Gromacs 2018 computer program. Results of the simulation demonstrated that at pH 6.8 polyethylene glycol facilitated vinpocetine solubilization and thus considerably enhanced its solubility in water. The data obtained show that the values of the energies of van der Waals interaction between vinpocetine and the polymer, as well as vinpocetine and water, both at a ratio of 1:2 and at a ratio of 1:5 are similar.
Keywords
Full Text:
PDFReferences
Golob S, Perry M, Lusi M, Chierotti MR, Grabnar I, Lassiani L, Voinovich D, Zaworotko MJ. Improving biopharmaceutical properties of vinpocetine through cocrystallization. J Pharm Sci. 2016;105(12):3626–3633. doi:10.1016/j.xphs.2016.09.017
Verma S, Rudraraju VS. A systematic approach to design and prepare solid dispersions of poorly water-soluble drug. AAPS Pharm Sci Tech. 2014;15(3):641–657. doi:10.1208/s12249-014-0093-z
Allawadi D, Singh N, Singh S, Arora S. Solid dispersions: a review on drug delivery system and solubility enhance-ment. Int J Pharm Sci Res. 2013;4(6);2094–2105. doi:10.13040/IJPSR.0975-8232.4(6).2094-05
Patil RM, Maniyar AH, Kale MT, Akarte AP, Baviskar DT. Solid dispersion: strategy to enhance solubility. Int J Pharm Sci Rev Res. 2011;8(2):66–73. doi:10.1007/s10973-016-5759-1
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17(9–10):486–495. doi:10.1016/j.drudis.2011.11.007
Good DJ, Rodríguez-Hornedo N. Solubility Advantage of Pharmaceutical Cocrystals. Crys Growth Des. 2009;9(5):2252–2264. doi:10.1021/cg801039j
Brough C, Williams III RO. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug de-livery. Int J Pharm. 2013;453(1):157–166. doi:10.1016/j.ijpharm.2013.05.061
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–1302. doi:10.1002/jps.2600600902
Akiladevi D, Shanmugapandian P, Jebasingh D, Sachinandan B. Preparation and evaluation of Paracetamol by solid dis-persion technique. Int J Pharm. 2011; 3(11): 88–191. doi:10.13040/IJPSR.0975-8232.5(10).4478-8
Dubey A, Kharia AA, Chatterjee DP. Enhancement of aque-ous solubility and dissolution of telmisartan using solid dispersion technique. IJPSR. 2014;5(10):4478–4485. doi:10.13040/IJPSR.0975-8232.5(10).4478-85
Biswal S, Sahoo J, Murthy PN, Giradkar RP. Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS Pharm Sci Tech. 2008;9(2):563–570. doi:10.1208/s12249-008-9079-z
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56. doi:10.1007/s40005-013-0058-3
Setyawan D, Setiawardani F, Amrullah Z, Sari R. PEG 8000 increases solubility and dissolution rate of quercetin in sol-id dispersion system. Marmara Pharm J. 2018;22(2):445–456. doi:10.12991/mpj.2018.63
Arroyo ST, Sansón Martín JA, Hidalgo García A. Molecular dynamics simulation of acetamide solvation using interac-tion energy components: Application to structural and ener-gy properties. Chem Phys. 2006;327(1):187-192. doi:10.1016/j.chemphys.2006.04.018
Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI force field: Coarse grained model for bio-molecular simulations. J Phys Chem B. 2007;111(27):7812–7824. doi:10.1021/jp071097f
Berendsen HJC, Postma JPM, Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81(8):3684–3690. doi:10.1063/1.448118
DOI: https://doi.org/10.15826/chimtech.2022.9.2.S11
Copyright (c) 2022 Yulia A. Polkovnikova, Tamara N. Glizhova, Naira V. Arutyunova, Natalya N. Sokulskaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







