Synthesis, in vitro and docking studies of 2-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-4(3H)-one derivatives as agents for the treatment of Alzheimer's disease
Abstract
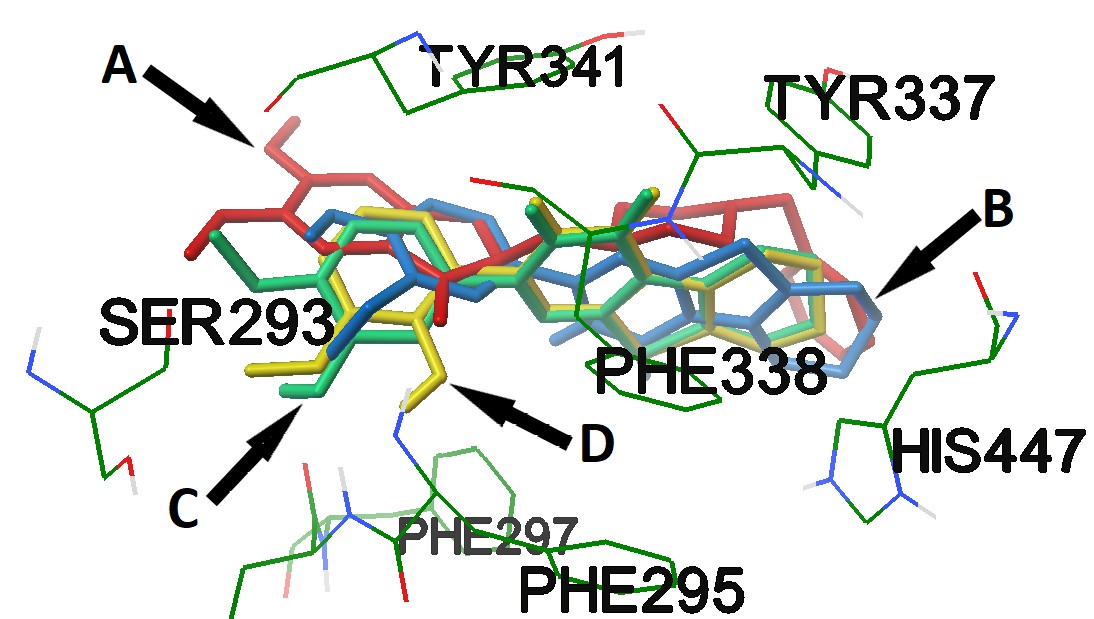
Alzheimer's disease is a chronic neurodegenerative disease, which is characterized mainly by a progressive decrease in intellectual abilities, memory impairment and a change in a person's personality. Unfortunately, there are practically no medicines that act on pathogenesis of Alzheimer's disease. The development of new highly effective medicines for the treatment of this pathology is an actual area of pharmaceutical research. The aim of this work is to search among 2-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-4(3H)-one effective compounds with an anticholinesterase and an antiamyloid activities. As a result, it was found that compounds 4d, 4e and 4f have the high anticholinesterase ability, which in their structure contain residues of hydroxy-methoxyphenyl fragments. Structures 4c, 4g, 4h, 4j, 4k, 4m, 4n and 4p showed slightly less activity, the effect of which did not differ statistically from that of Donepezil. Compounds 4c, 4e, 4k and 4m have the greatest ability to inhibit the formation of the amyloid, comparable to GV-971. It should be noted that the molecular docking data are consistent with the results of the determination of the anticholinesterase activity of the studied compounds obtained in vitro. Thus, the prospects for future studies of these compounds in the possibility of creating a pharmaceutical active substance for the treatment of neurodegenerative diseases have been revealed.
Keywords
Full Text:
PDFReferences
Weber SA, Patel RK, Lutsep HL. Cerebral amyloid angiopathy: diagnosis and potential therapies. Expert Rev Neurother. 2018;18(6):503–513. doi:10.1080/14737175.2018.1480938
Sağlık BN, Osmaniye D, Acar Çevik U, Levent S, Kaya Çavuşoğlu B, Özkay Y, Kaplancıklı ZA. Design, synthesis, and structure-activity relationships of thiazole analogs as anticholinesterase agents for alzheimer’s disease. Mol. 2020;25(18):4312. doi:10.3390/molecules25184312
Bacalhau P, San Juan AA, Marques CS, Peixoto D, Goth A, Guarda C, Silva M, Arantes S, Caldeira AT, Martins R, Burke AJ. New cholinesterase inhibitors for Alzheimer’s disease: Structure Activity Studies (SARs) and molecular docking of isoquinolone and azepanone derivatives. Bioorg Chem. 2016;67:1–8. doi:10.1016/j.bioorg.2016.05.004
Riswanto FDO, Rawa MSA, Vikneswaran M, Salin NH, Istyastono EP, Hariono M, Wahab HA. Anti-cholinesterase activity of chalcone derivatives: synthesis, in vitro assay and molecular docking study. Medicinal Chemistry 2021;17(5):442–452. doi:10.2174/1573406415666191206095032
Jang C, Yadav DK, Subedi L, Venkatesan R, Venkanna A, Afzal S, Lee E, Yoo J, Ji E, Kim SY, Kim m. Identification of novel acetylcholinesterase inhibitors designed by pharmacophore-based virtual screening, molecular docking and bioassay. Sci Rep. 2018;8:14921. doi:10.1038/s41598-018-33354-6
Hatice ED. Pyrimidines: Molecular docking and inhibition studies on carbonic anhydrase and cholinesterases. Biotechnol Appl Biochem. 2022. doi:10.1002/bab.2329
Bharate JB, Ådén J, Gharibyan A, Adolfsson DE, Jayaweera SW, Singh P, Vielfort K, Tyagi M, Bonde M, Bergström S, Olofsson A, Almqvist F. K2S2O8-mediated coupling of 6-amino-7-aminomethyl-thiazole-pyridine with aldehydes to construct amyloid affecting pyrimidine-fused thiazolino-2-pyridones. Org Biomol Chem. 2021;19;9758–9772. doi:10.1039/D1OB01580J
Bortolami M, Pandolfi F, Tudino V, Messore A, Madia VN, De Vita D, Di Santo R, Costi R, Romeo I, Alcaro S, Colone M, Stringaro A, Espargaro A, Sabate R, Scipione l. New pyrimidine and pyridine derivatives as multitarget cholinesterase inhibitors: design, synthesis, and in vitro and in cellulo evaluation. ACS Chem Neurosci. 2021;12(21):4090–4112. doi:10.1021/acschemneuro.1c00485
Javed MA, Ashraf N, Saeed Jan M, Mahnashi MH, Alqahtani YS, Alyami BA, Alqarni AO, Asiri YI, Ikram M, Sadiq A, Rashid U. Structural modification, in vitro, in vivo, ex vivo, and in silico exploration of pyrimidine and pyrrolidine cores for targeting enzymes associated with neuroinflammation and cholinergic deficit in Alzheimer’s Disease. ACS Chem Neurosci. 2021;12(21):4123–4143. doi:10.1021/acschemneuro.1c00507
Manzoor S, Prajapati SK, Majumdar S, Raza MK, Gabr MT, Kumar S, Pal K, Rashid H, Kumar S, Krishnamurthy S, Hoda N. Structural modification, in vitro, in vivo, ex vivo, and in silico exploration of pyrimidine and pyrrolidine cores for targeting enzymes associated with neuroinflammation and cholinergic deficit in Alzheimer’s disease. Eur J Med Chem. 2021;215:113224. doi:10.1016/j.ejmech.2021.113224
Shahid NM, Azam Khan J, Kazmi I, Rashid U. Design, synthesis, and bioevaluation of indole core containing 2-arylidene derivatives of thiazolopyrimidine as multitarget inhibitors of cholinesterases and monoamine oxidase A/B for the treatment of Alzheimer disease. ACS Omega. 2022;7(11):9369–9379. doi:10.1021/acsomega.1c06344
Mariki A, Anaeigoudari A, Zahedifar M, Pouramiri B. design, green synthesis, and biological evaluation of new substituted tetrahydropyrimidine derivatives as acetylcholinesterase inhibitors. Polycycl Aromat Compd. 2021. doi:10.1080/10406638.2021.1933102
Kumar V, Kumar B, Ranjan Dwivedi A, Mehta D, Kumar N, Bajaj B, Arora T, Prashar V, Parkash J, Kumar V. Design, synthesis and evaluation of O-Pentyne substituted diphenylpyrimidines as monoamine oxidase and acetylcholinesterase inhibitors. Chem Sel. 2020;5:8021. doi:10.1002/slct.202002425
Zribi L, Pachòn-Angona I, Bautista-Aguilera ÒM, Diez-Iriepa D, Marco-Contelles J, Ismaili L, Iriepa I, Chabchoub F. Triazolopyridopyrimidine: A new scaffold for dual-target small molecules for Alzheimer’s disease therapy. Mol. 2020;25(14):3190. doi:10.3390/molecules25143190
Chiriapkin AS, Kodonidi IP, Pozdnyakov DI, Zolotych DS. Synthesis and QSAR of new azomethine derivatives as agents for the treatment of Alzheimer's disease. Pharmacologyonline. 2021;3:563–584. https://pharmacologyonline.silae.it/files/archives/2021/vol3/PhOL_2021_3_A062_Chiriapkin.pdf
Amawi H, Hussein N, Boddu SHS, Karthikeyan C, Williams FE, Ashby CRJ, Raman D, Trivedi P, Tiwari AK. Novel Thienopyrimidine Derivative, RP-010, Induces β-Catenin fragmentation and is efficacious against prostate Cancer Cells. Cancers. 2019;11:711. doi:10.3390/cancers11050711
Amawi H, Karthikeyan C, Pathak R, Hussein N, Christman R, Robey R, Ashby CR, Trivedi P, Malhotra A, Tiwari AK. Thienopyrimidine derivatives exert their anticancer efficacy via apoptosis induction, oxidative stress and mitotic catastrophe. Eur J Med Chem. 2017;138:1053–1065. doi:10.1016/j.ejmech.2017.07.028
Wang YD, Johnson S, Powell D, McGinnis JP, Miranda M, Rabindran SK. Inhibition of tumor cell proliferation by thieno[2,3-d]pyrimidin-4(1H)-one-based analogs. Bioorg Med Chem Lett. 2005;15(16):3763–3766. doi:10.1016/j.bmcl.2005.05.127
Zhang Y, Luo L, Han C, Lv H, Chen D, Shen G, Ye F. Design, Synthesis, and Biological Activity of Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine Derivatives as Anti-Inflammatory Agents. Mol. 2017;22(11):1960. doi:10.3390/molecules22111960
Ouyang L, Zhang L, Liu J, Fu L, Yao D, Zhao Y, Zhang S, Wang G, He G, Liu B. Discovery of a small-molecule bromodomain-containing protein 4 (BRD4) inhibitor that induces AMP-activated protein kinase-modulated autophagy-associated cell death in breast cancer. J Med Chem. 2017;60(24):9990–10012. doi:10.1021/acs.jmedchem.7b00275
El-Mekabaty A, Fouda AE-AS, Shaaban IEI. Convenient synthesis of functionalized thieno[2,3-d]pyrimidine-4-ones and thieno[2,3-b]pyridine-4-ones bearing a pyridine moiety with anticipated antioxidant activity. J Heterocyclic Chem. 2020;57:2928–2935. doi:10.1002/jhet.4002
Kawade DP, Chaple DR, Khedekar PB. Synthesis, physicochemical characterization and analgesic evaluation of some new thieno[2,3-D]Pyrimidin-4(3H)-one derivatives. Int J Pharm Chem Anal. 2020;7(1):32–38. doi:10.18231/j.ijpca.2020.005
Pal K, Raza MK, Legac J, Ataur Rahman M, Manzoor S, Rosenthal PJ, Hoda N. Design, synthesis, crystal structure and anti-plasmodial evaluation of tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives. RSC Med Chem. 2021;6:970–981. doi:10.1039/d1md00038a
Shi-Ben W, Xian-Qing D, Yan Z, Yan-Ping Y, Zhe-Shan Q, Li-Ping G. Synthesis and evaluation of anticonvulsant and antidepressant activities of 5-alkoxytetrazolo[1,5-c]thieno[2,3-e]pyrimidine derivatives. Eur J Med Chem. 2012;56:139–144. doi:10.1016/j.ejmech.2012.08.027
Ghayour F, Mohammad S, Mohammad R, Ghashang M. ZnO-CeO2 nanocomposite: efficient catalyst for the preparation of thieno[2,3-d]pyrimidin-4(3H)-one derivatives. Main Group Metal Chem. 2018;41(1–2):21–26. doi:10.1515/mgmc-2017-0038
Dzhavakhishvili SG, Gorobets NYu, Musatov VI, Desenko SM, Paponov BV. Three possible products from the reactions of gewald’s amide with aromatic aldehydes. J Heterocycl Chem. 2008;45(2):573–577. doi:10.1002/jhet.5570450243
Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. 2012;55(22):10282–10286. doi:10.1021/jm300871x
Chiriapkin AS, Kodonidi IP, Larsky MV. Targeted Synthesis and Analysis of Biologically Active Azomethine Derivatives of 2-amino-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxamide. Drug Dev Regist. 2021;10(2):25–31. doi:10.33380/2305-2066-2021-10-2-25-31
Chiriapkin A, Kodonidi I, Ivchenko A, Smirnova L. Synthesis and Prognosis of Anti-inflammatory Activity of 2-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-4(3H)-one. Bull Sci Pract. 2021;7(12):25–33. doi:10.33619/2414-2948/73/02
Wang W, Zhao C, Zhu D, Gong G, Du. W. Inhibition of amyloid peptide fibril formation by gold-sulfur complexes. J Inorg Biochem. 2017;171:1–9. doi:10.1016/j.jinorgbio.2017.02.021
Jończyk J, Godyń J, Stawarska E, Marak-Mlodawska B, Jelen M, Pluta K, Malawska B. Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives-Anticancer and Cholinesterase-Inhibiting Activity. Mol. 2020;25(11):2604. doi:10.3390/molecules25112604
Mahgoub N, Alexopoulos GS. Amyloid Hypothesis: Is There a Role for Antiamyloid Treatment in Late-Life Depression? Am J Geriatr Psychiatry. 2016;24(3):239–247. doi:10.1016/j.jagp.2015.12.003
Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer's disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2020;16(11):1553–1560. doi:10.1016/j.jalz.2019.09.075
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (NY). 2019;5:272–293. doi:10.1016/j.trci.2019.05.008
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain. 2018;141(7):1917–1933. doi:10.1093/brain/awy132
DOI: https://doi.org/10.15826/chimtech.2022.9.2.04
Copyright (c) 2022 Alexey S. Chiriapkin, Ivan P. Kodonidi, Dmitry I. Pozdnyakov, Alexander A. Glushko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







