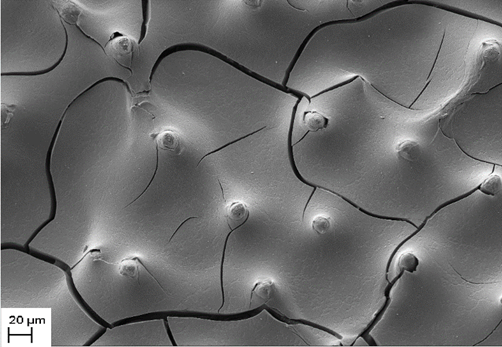
Synthesis of polycalciumphenylsiloxane and composites based on the skeleton of a sea urchin with the resulting polymer
Abstract
Keywords
Full Text:
PDFReferences
Coradin T, Lopes PJ. Biogenic Silica Patterning: Simple Chemistry or Subtle Biology? ChemBioChem. 2003;3:1–9. doi:10.1002/cbic.200390044
Sanchez C, Arribart H, Madeleine GG. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nature Mater. 2005;4:277–288. doi:10.1038/nmat1339
Michalczyk MJ, Farneth WE, Vega АJ. High temperature stabilization of crosslinked siloxanes glasses. Chem Mater. 1993;5(12):1687–1689. doi:10.1021/cm00036a001
Eguchi K, Zank GA. Silicon Oxycarbide Glasses Derived from Polymer Precursors. J Sol-Gel Sci Technol. 1998;13:945–949. doi:10.1023/A:1008639727164
Choudhary R, Kumar Venkatraman S, Bulygina I, Senatov F, Kaloshkin S, Anisimova N, Kisilevskiy M, Knyazeva M, Kukui D, Walther F, Swamiappan S. Biomineralization, dissolution and cellular studies of silicate bioceramics prepared from eggshell and rice husk. Mater Sci Eng C. 2021;118:111456. doi:10.1016/j.msec.2020.111456
Levitsky MM, Zavin BG, Bilyachenko AN. Chemistry of metallasiloxanes. Current trends and new concepts. Russ Chem Rev. 2007;76(9):907–926. doi:10.1070/RC2007v076n09ABEH003691
Prockop DJ, Fertala A. The collagen fibril: the almost crystalline structure. J Struct Biol. 1998;122:111–118. doi:10.1006/jsbi.1998.3976
Erlich H, Brunner Е, Simon Р et al. Calcite reinforced silica–silica joints in the biocomposite skeleton of deep-sea glass sponges. Adv Funсt Mater. 2011;21:3473–3481. doi:10.1002/adfm.201100749
Kuzmina NP, Chechernikova MV, Martynenko LI. Calcium acetylacetonates. J Inorg Chem. 1990;35(11):2776–2780.
Shapkin NP, Kapustina AA, Dombai NV, Libanov VV, Khalchenko IG, Gardionov SV, Gribova VV. Synthesis and physicochemical characteristics of polymolybdenum(VI) phenylsiloxanes by means of different methods. Polym Bull. 2020;77(3):1177–1190. doi:10.1007/s00289-019-02790-3
Morozova NB, Zharkova GI et al. Synthesis and physical-chemical study of β-diketonates of alkaline earth metals. Novosibirsk; 1989. 28 p.
Shapkin NP, Kapustina AA, Gardionov SV, Khalchenko IG, Libanov VV, Tokar EA. Studies of Interaction of Polyphenylsiloxane with Vanadyl Bis-Acetylacetonate. Silicon. 2019;11(5):2261–2266. doi:10.1007/s12633-017-9551-z
Berman A, Addadi L, Kvick Å, Leiserowitz L, Nelson M, Weiner S. Intercalation of sea urchin proteins in calcite: study of a crystalline composite material. Sci. 1990;250:664–667. doi:10.1126/science.250.4981.664
Seto J, Ma Y, Davis S et al. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc Nat Acad Sci. 2012;109:3699–3704. doi:10.1073/pnas.1109243109
Shapkin NP, Papynov EK, Panasenko AE, Khalchenko IG, Mayorov VYu, Drozdov AL, Maslova NV, Buravlev IYu. Synthesis of porous biomimetic composites: A sea urchin skeleton used as a template. Appl Sci. 2021;11;19:8897. doi:10.3390/app11198897
DOI: https://doi.org/10.15826/chimtech.2022.9.2.S6
Copyright (c) 2022 Nikolai P. Shapkin, Irina G. Khalchenko, Kvan H. Kim, Evgeniy K. Papynov, Alexander N. Fedorets, Natalia V. Maslova, Michael I. Balanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







